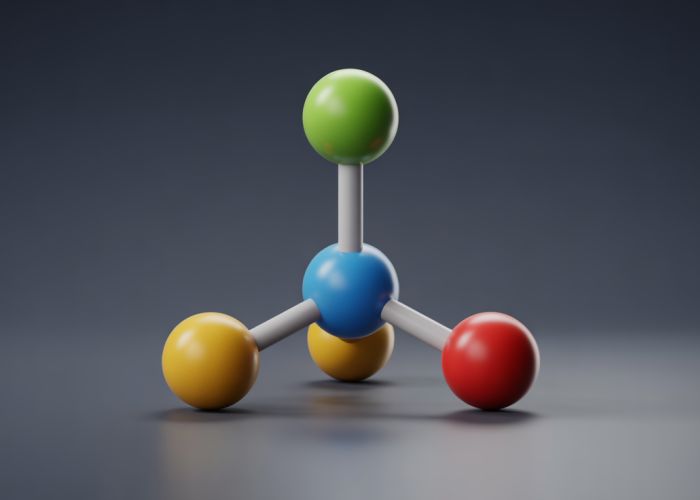
Ammonia (NH3), a common chemical compound, exemplifies the trigonal pyramid structure, a fundamental concept in VSEPR theory. Molecular geometry, specifically this structure, influences a molecule’s physical and chemical properties. Understanding this arrangement is vital in fields such as organic chemistry and is easily visualized using online molecular modeling software.
Understanding the Trigonal Pyramid Structure: A Comprehensive Guide
This guide provides a clear and detailed explanation of the trigonal pyramid structure, covering its key features, characteristics, and relevant examples.
Defining the Trigonal Pyramid Structure
The trigonal pyramid structure is a geometric shape characterized by its triangular base and three triangular faces that converge at a single point, known as the apex. It’s a fundamental structure found in various fields, from chemistry to architecture. Understanding its properties is crucial for comprehending molecular geometry and other related concepts.
Key Characteristics
- Base: A triangle, typically equilateral, but it can also be isosceles or scalene.
- Faces: Three triangular faces that connect the base to the apex.
- Apex: The point where the three triangular faces meet.
- Vertices: Four vertices (corners) – three at the base and one at the apex.
- Edges: Six edges – three forming the base and three connecting the base to the apex.
- Symmetry: Trigonal pyramids generally exhibit C3v symmetry (a threefold rotational axis and three vertical mirror planes).
Exploring the Geometry of the Trigonal Pyramid
The precise angles and dimensions within a trigonal pyramid structure are important. While a perfect trigonal pyramid (all sides and angles equal) is a theoretical ideal, real-world examples often show slight variations.
Ideal Geometry vs. Real-World Deviations
- Ideal Trigonal Pyramid: In a perfectly regular trigonal pyramid, all four faces are equilateral triangles.
- Bond Angles (Ideal): The bond angles are typically around 109.5° when observed in molecules with this shape due to VSEPR theory predictions. However, this can vary based on the atoms involved.
- Deviations: Deviations from the ideal geometry can occur due to differences in the electronegativity of atoms, lone pairs of electrons, or steric hindrance (the spatial arrangement of atoms hindering rotation).
Examples in Chemistry: Molecular Geometry
The trigonal pyramid structure is most commonly encountered when discussing molecular geometry in chemistry. Molecules adopt this shape when a central atom is bonded to three other atoms and has one lone pair of electrons. This follows the VSEPR (Valence Shell Electron Pair Repulsion) theory, which dictates that electron pairs (both bonding and non-bonding) around a central atom will arrange themselves to minimize repulsion.
Key Examples of Trigonal Pyramidal Molecules
-
Ammonia (NH3): Nitrogen (N) is the central atom, bonded to three hydrogen (H) atoms and possessing one lone pair of electrons. The lone pair repels the bonding pairs, causing the bond angle to be slightly smaller than the ideal tetrahedral angle (approximately 107°).
Property Value Central Atom Nitrogen (N) Bonding Atoms Hydrogen (H) Lone Pairs 1 Approximate Bond Angle ~107° -
Phosphorus Trichloride (PCl3): Phosphorus (P) is the central atom, bonded to three chlorine (Cl) atoms and possessing one lone pair.
-
Hydronium Ion (H3O+): Oxygen (O) is the central atom, bonded to three hydrogen atoms and possessing one lone pair, giving it a trigonal pyramidal shape.
VSEPR Theory and the Trigonal Pyramid
The VSEPR theory is vital for predicting the trigonal pyramid structure. The theory predicts that four electron groups around a central atom will arrange themselves in a tetrahedral geometry. If one of these four groups is a lone pair, the molecular shape becomes trigonal pyramidal. The greater repulsive force of the lone pair pushes the bonding pairs closer together, reducing the bond angles compared to a perfect tetrahedron.
Real-World Applications Beyond Chemistry
While primarily associated with chemistry, the trigonal pyramid form appears in other fields, though less frequently as a perfectly replicated structure. Elements of the shape can be found in architectural designs and even in some crystalline structures.
Architectural Considerations
Architectural uses of a trigonal pyramid shape are more common in decorative elements or as a module in larger structures, rather than as a standalone building shape. The structural stability of a true trigonal pyramid can be challenging to achieve on a large scale, so variations or modified forms are usually employed.
Crystalline Structures
While true trigonal pyramidal arrangements of atoms might not be as prevalent in large crystal lattices, localized motifs exhibiting this geometry can be found in certain complex crystalline materials. Analyzing these local arrangements is crucial for understanding the overall properties of the material.
FAQs About Trigonal Pyramid Structures
Here are some frequently asked questions about trigonal pyramid structures, to help you understand them better.
What exactly defines a trigonal pyramid structure?
A trigonal pyramid structure is a geometric arrangement of atoms where four atoms are positioned at the corners of a triangular pyramid, also known as a tetrahedron. One atom sits at the apex (top point), and the other three form the triangular base.
How does a trigonal pyramid structure differ from a tetrahedral structure?
While both are based on a tetrahedron, a key difference lies in their overall shape and symmetry, usually determined by the presence of lone pairs of electrons. The presence of a lone pair can distort the symmetrical tetrahedral arrangement into the trigonal pyramid structure.
What are some common examples of molecules with a trigonal pyramid structure?
Ammonia (NH3) is a very common example. The nitrogen atom sits at the apex with the three hydrogen atoms forming the triangular base of the trigonal pyramid structure.
Is the trigonal pyramid structure always a perfect pyramid?
No, due to differing electronegativities and the repulsion of lone pairs of electrons on the central atom, the angles and bond lengths in a trigonal pyramid structure can be distorted, leading to a shape that deviates from a perfect pyramid.
Hopefully, this simple guide cleared up any confusion about the trigonal pyramid structure! Now you can confidently identify and describe molecules with this interesting shape. Happy studying!