Understanding the behavior of elements often begins with grasping their electron configuration. Indeed, the periodic table itself arranges elements based on these electronic structures. Now, when considering how ionization energy affects element stability, we can examine the electron configuration of sodium. Specifically, electron configuration sodium illustrates how easily it gives up one electron to achieve a full outer shell, resembling the more stable noble gas configuration of neon. Such an understanding is crucial for chemists to create, evaluate, and understand reactions.
Did you sprinkle salt on your food today? If so, you’ve already encountered sodium, one of the most common elements on Earth.
But sodium is more than just a seasoning; it’s a fundamental building block of our world, and its behavior is dictated by its electron configuration.
But what exactly is electron configuration, and why should you care?
Electron configuration unlocks an element’s properties, predicting how it will interact with other elements and form the myriad compounds that shape our lives. Understanding it helps us to predict and explain the chemical behaviour of elements.
This article aims to demystify the electron configuration of sodium (Na) in a clear and accessible way.
We will explore the arrangement of its electrons, revealing the secrets behind its unique reactivity.
The Atomic Foundation: A Quick Look at Sodium’s Structure
Before diving into the electron configuration, let’s briefly revisit sodium’s atomic structure.
Each sodium atom contains a nucleus with 11 protons and typically 12 neutrons.
Orbiting this nucleus are 11 electrons, arranged in specific energy levels and orbitals.
It is the arrangement of these electrons that determines sodium’s properties.
Did you sprinkle salt on your food today? If so, you’ve already encountered sodium, one of the most common elements on Earth.
But sodium is more than just a seasoning; it’s a fundamental building block of our world, and its behavior is dictated by its electron configuration.
But what exactly is electron configuration, and why should you care?
Electron configuration unlocks an element’s properties, predicting how it will interact with other elements and form the myriad compounds that shape our lives. Understanding it helps us to predict and explain the chemical behaviour of elements.
This article aims to demystify the electron configuration of sodium (Na) in a clear and accessible way.
We will explore the arrangement of its electrons, revealing the secrets behind its unique reactivity.
The Atomic Foundation: A Quick Look at Sodium’s Structure
Before diving into the electron configuration, let’s briefly revisit sodium’s atomic structure.
Each sodium atom contains a nucleus with 11 protons and typically 12 neutrons.
Orbiting this nucleus are 11 electrons, arranged in specific energy levels and orbitals.
It is the arrangement of these electrons that determines sodium’s properties.
Now, to truly grasp how these electrons orchestrate sodium’s behavior, we need to establish a solid foundation. Let’s explore the fundamental principles that govern electron configuration. These are the building blocks upon which our understanding of sodium—and indeed, all elements—will rest.
Electron Configuration Fundamentals: Building Blocks for Understanding
To navigate the world of electron configuration, it’s essential to understand the core concepts that govern it. Think of these as the essential tools in your chemistry toolkit.
The Mighty Electron
At the heart of it all lies the electron, a negatively charged particle that orbits the nucleus of an atom. It’s crucial to remember that electrons are not just static objects.
They are dynamic participants in chemical reactions.
The way electrons are arranged dictates how an atom will interact with others. It determines the types of bonds it will form. It also determines the compounds it will create.
Electrons are the actors on the stage of chemical reactions, and understanding them is key to understanding chemistry itself.
Decoding the Atomic Number
Each element has a unique atomic number, which represents the number of protons in its nucleus. In a neutral atom, the number of protons is equal to the number of electrons.
For sodium (Na), the atomic number is 11.
This tells us that a neutral sodium atom has 11 electrons to arrange in its electron configuration. This number is our starting point, our guiding star, in figuring out where each electron resides.
Think of the atomic number as the element’s unique identification code, instantly telling you how many electrons you need to account for.
Energy Levels and Shells: Electron Housing
Electrons don’t just orbit the nucleus randomly; they occupy specific energy levels, often visualized as shells surrounding the nucleus.
These shells are quantized, meaning electrons can only exist at certain energy levels, much like steps on a staircase.
The first energy level (closest to the nucleus) can hold up to 2 electrons. The second can hold up to 8, and the third can hold up to 18 (though the rules get a bit more nuanced as we move to higher energy levels).
Think of each shell as a floor in an apartment building, with electrons as the residents. Each floor has a limited capacity.
Understanding these energy levels is key to understanding how electrons arrange themselves around the nucleus.
The Power of Valence Electrons
Of all the electrons in an atom, the ones in the outermost shell are the most influential. These are the valence electrons.
These electrons are primarily responsible for an element’s chemical behavior.
It’s these valence electrons that participate in bonding with other atoms.
An element’s reactivity is directly tied to the number and arrangement of its valence electrons. In the case of sodium, as we’ll see, its single valence electron is the key to its reactivity.
To truly understand the power of these principles, let’s apply them directly to our element of interest. We’ll meticulously unpack how sodium’s atomic number dictates its electron configuration, piece by piece.
Sodium’s Electron Configuration: A Step-by-Step Guide
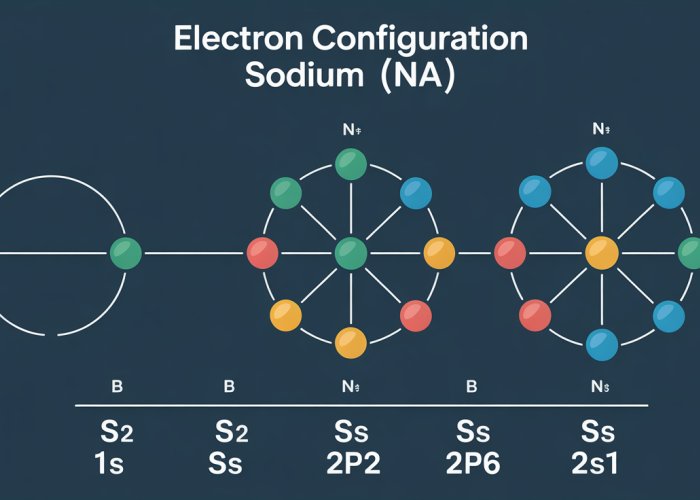
Unlocking sodium’s chemical secrets begins with understanding its electron configuration. This arrangement, denoted as 1s²2s²2p⁶3s¹, might seem like a cryptic code at first glance. However, by systematically breaking it down, we can reveal the fundamental reasons behind sodium’s behavior.
Atomic Number as the Key
The atomic number is the master key. Sodium, with an atomic number of 11, has 11 protons in its nucleus. In a neutral atom, this also means it has 11 electrons orbiting that nucleus.
These 11 electrons must be arranged according to the rules we’ve established, filling the lowest energy levels first. This principle is your roadmap to unlocking electron configurations of all elements.
Deciphering the Code: Shells and Orbitals
Let’s dissect the electron configuration 1s²2s²2p⁶3s¹ term by term. Each term represents a specific energy level (shell) and orbital, with the superscript indicating the number of electrons occupying that orbital.
-
1s²: The first energy level (n=1) has only one s orbital. It can hold a maximum of two electrons. In sodium, this orbital is completely filled with two electrons.
-
2s²: The second energy level (n=2) contains an s orbital, which, as with the first energy level, is fully occupied with two electrons.
-
2p⁶: The second energy level also includes three p orbitals (px, py, and pz), collectively holding up to six electrons. Sodium completely fills these p orbitals in its second shell.
-
3s¹: Finally, we reach the third energy level (n=3). Here, we find a single s orbital. Sodium has only one electron in this orbital. It’s this lone electron in the outermost shell that dictates much of sodium’s reactivity.
Visualizing Electron Placement
While the notation 1s²2s²2p⁶3s¹ is precise, it can be helpful to visualize the electron arrangement. Diagrams, such as orbital box diagrams or Bohr models, can provide a clearer picture of electron placement.
These diagrams visually represent energy levels and orbitals as boxes or orbits, with arrows indicating individual electrons. Using these visual aids can greatly improve understanding, particularly when learning electron configuration for the first time. Embrace these tools—they can illuminate the sometimes-abstract world of quantum mechanics and electron structure.
To truly understand the implications of its configuration, we must now turn our attention to sodium’s valence electrons, the key players in its chemical behavior. These outermost electrons dictate how sodium interacts with other elements, influencing its reactivity and stability in the chemical world.
The Significance of Valence Electrons: Reactivity and Stability
Valence Electrons: The Key to Chemical Reactions
Valence electrons, those residing in the outermost shell of an atom, are the gatekeepers of chemical reactivity. These are the electrons involved in forming chemical bonds with other atoms.
The number of valence electrons an atom possesses determines how it will interact with other atoms. For example, an atom with only one valence electron, like sodium, has a strong tendency to lose that electron.
The Octet Rule: A Quest for Stability
Atoms strive for stability, and this drive is largely governed by the octet rule. The octet rule states that atoms tend to gain, lose, or share electrons in order to achieve a full outer shell of eight valence electrons, mimicking the stable electron configurations of noble gases.
Sodium, with its electron configuration of 1s²2s²2p⁶3s¹, has only one valence electron in its 3s orbital.
To achieve a full outer shell, sodium has two options: gain seven more electrons, or lose its single valence electron.
The latter, losing one electron, is energetically more favorable.
Sodium’s Transformation: From Atom to Ion (Na+)
When sodium loses its single valence electron, it transforms into a positively charged ion, Na+.
By losing this electron, the third shell is emptied, and sodium now has a full second shell, containing eight electrons (2s²2p⁶).
This resulting electron configuration, identical to that of neon, a noble gas, is highly stable.
The process of losing an electron and becoming an ion is how sodium achieves a stable octet.
The Noble Gas Connection: Models of Stability
Noble gases, such as helium, neon, and argon, are renowned for their exceptional stability and inertness. This is because they already possess a full outer shell of eight valence electrons (except for helium, which has two).
Their stable electron configurations make them largely unreactive. Atoms like sodium strive to attain this noble gas configuration, even if it means gaining or losing electrons to achieve it.
This drive towards stability underlines much of chemical reactivity.
Sodium’s Place on the Periodic Table: A Reflection of its Configuration
The periodic table is not just a list of elements; it’s a map that reflects the underlying electron configurations of atoms.
Sodium resides in Group 1 (also known as the alkali metals) of the periodic table.
Elements in the same group share similar chemical properties because they have the same number of valence electrons.
All alkali metals, like sodium, have one valence electron and tend to lose it to form +1 ions.
Sodium’s position on the periodic table is a direct consequence of its electron configuration and its tendency to readily lose one electron, defining its characteristic chemical behavior.
By losing this electron, the third shell is emptied, leaving a stable outer shell with eight electrons (from the second shell). This transformation highlights the power of electron configuration in predicting ionic behavior. But what does all of this mean in the real world? How does sodium’s electron configuration influence the properties and applications of this ubiquitous element?
Real-World Applications: Why Sodium’s Configuration Matters
Sodium’s electron configuration isn’t just an abstract concept confined to textbooks and laboratories. It’s the key that unlocks our understanding of sodium’s observable properties and widespread applications. The drive to achieve a stable octet, dictated by its electron configuration, dictates how it interacts with the world around it.
Reactivity and Conductivity: A Direct Consequence
Sodium is known for its high reactivity. This is a direct consequence of its single valence electron. It readily gives up this electron to form chemical bonds with other elements, particularly nonmetals like chlorine and oxygen.
This eagerness to shed its valence electron also contributes to its excellent electrical conductivity. Metals, in general, conduct electricity because their valence electrons are relatively free to move throughout the material. Sodium, with its loosely held valence electron, is no exception. This is why, although not as common as copper or aluminum due to its reactivity, sodium finds specialized applications in electrical conductors.
Sodium Compounds: Essential Components of Modern Life
The compounds formed by sodium are incredibly diverse and essential to many aspects of modern life. Let’s explore just a few examples:
-
Sodium Chloride (NaCl): More commonly known as table salt, sodium chloride is vital for human health, food preservation, and various industrial processes. It is used in everything from seasoning our meals to manufacturing plastics and pharmaceuticals.
-
Sodium Bicarbonate (NaHCO₃): Baking soda, or sodium bicarbonate, is a common household ingredient used in baking, cleaning, and even as a remedy for indigestion. Its ability to release carbon dioxide gas makes it a crucial component in leavening agents.
-
Sodium Hydroxide (NaOH): Also known as lye or caustic soda, sodium hydroxide is a highly alkaline substance used in the production of paper, soaps, detergents, and various chemicals. It’s a powerful base with a wide range of industrial applications.
-
Sodium Carbonate (Na₂CO₃): Known as soda ash or washing soda, sodium carbonate is used in the manufacture of glass, detergents, and other chemicals. It is also used as a water softener and a cleaning agent.
These examples demonstrate the critical role that sodium compounds play in our daily lives. The unique properties of each compound are directly related to the way sodium interacts with other elements, a behavior rooted in its fundamental electron configuration.
Laying the Foundation for Chemical Understanding
Understanding sodium’s electron configuration is more than just memorizing a sequence of numbers and letters. It’s a gateway to grasping more complex chemical concepts. Once you understand why sodium behaves the way it does, you can start to predict the behavior of other elements and compounds.
The periodic table becomes less of a daunting chart and more of a map, revealing predictable trends in chemical properties based on electron configurations. Concepts like electronegativity, ionization energy, and bond polarity become clearer when viewed through the lens of electron arrangement.
Frequently Asked Questions: Sodium’s Electron Configuration
Here are some common questions about sodium’s electron configuration and how it works. Hopefully, this clarifies any confusion!
Why is understanding sodium’s electron configuration important?
Knowing the electron configuration of sodium, or any element, helps us predict its chemical behavior. The outer electrons are the ones involved in bonding, so understanding their arrangement is crucial for understanding reactivity. It’s foundational for many chemistry concepts.
What does "1s² 2s² 2p⁶ 3s¹" actually mean for the electron configuration sodium?
This notation tells you how the electrons are arranged around the sodium atom’s nucleus. 1s², for example, means two electrons occupy the 1s orbital. 2s² and 2p⁶ represent two and six electrons in the second energy level, respectively. Finally, 3s¹ signifies one electron in the outermost, third energy level.
How does sodium achieve a stable electron configuration?
Sodium achieves a more stable electron configuration by losing the single electron in its outer 3s¹ orbital. By losing this electron, it forms a positively charged sodium ion (Na+) and attains the same electron configuration as neon, a noble gas. This loss results in a full outer shell, making it more stable.
Is sodium reactive due to its electron configuration?
Yes, the electron configuration of sodium with its single valence electron (3s¹) makes it highly reactive. Sodium readily donates this electron to achieve a more stable, full outer shell. This eagerness to lose the electron is why sodium reacts vigorously with elements like chlorine and oxygen.
So, that’s the scoop on electron configuration sodium! Hopefully, things are a bit clearer now. Go forth and conquer those chemistry concepts!