The atom’s structure, a fundamental concept in physics, underwent significant refinement through the Rutherford atomic model. Ernest Rutherford, the pioneering physicist, proposed this revolutionary model. Alpha particle scattering provided the experimental evidence that underpinned the model’s development. The Geiger-Marsden experiment, conducted under Rutherford’s guidance, delivered crucial data about the atom’s composition. This article explores the key elements of the rutherford atomic model, examining its strengths and limitations within the broader context of atomic theory.
The atom, derived from the Greek word atomos meaning indivisible, represents the fundamental building block of all matter in the universe. Understanding its structure and behavior is paramount to comprehending the properties of everything around us, from the simplest elements to the most complex biological molecules.
From chemistry and materials science to medicine and energy production, the atom’s secrets underpin countless scientific and technological advancements.
A Journey Through Atomic Models
Our understanding of the atom did not emerge overnight. It is the result of centuries of scientific inquiry, with each model building upon the successes and addressing the limitations of its predecessors.
Early philosophical ideas about indivisible particles gradually gave way to more scientific models as experimental techniques advanced.
From Dalton to Thomson
John Dalton’s atomic theory in the early 19th century laid the groundwork, proposing that elements are composed of identical atoms that combine in simple ratios to form compounds.
Later, the discovery of subatomic particles, most notably the electron by J.J. Thomson, necessitated a revision of Dalton’s model.
Thomson proposed the "plum pudding" model, envisioning the atom as a sphere of positive charge with negatively charged electrons embedded within it. While a significant step forward, the plum pudding model lacked experimental support and failed to explain certain observed phenomena.
The quest for a more accurate depiction of atomic structure continued.
The Rutherford Revolution: A Thesis
This brings us to the pivotal work of Ernest Rutherford and his groundbreaking Gold Foil Experiment. Through meticulous experimentation and insightful interpretation, Rutherford challenged the prevailing atomic model and ushered in a new era of atomic understanding.
The Rutherford atomic model, derived from the Gold Foil Experiment, revolutionized atomic structure understanding by introducing a dense, positively charged atomic nucleus.
His model not only explained the experimental results but also provided a framework for future advancements in atomic theory and quantum mechanics, forever changing our perspective on the fundamental nature of matter.
The quest for a more accurate depiction of atomic structure continued. The scientific community recognized the need to refine or even replace existing models as new experimental evidence emerged, challenging established theories.
The Pre-Rutherford Era: Thomson’s Plum Pudding Model
Before Rutherford’s revolutionary Gold Foil Experiment, the prevailing model of the atom was J.J. Thomson’s "plum pudding" model. Proposed in 1904, this model represented a significant step beyond Dalton’s indivisible atom, incorporating the newly discovered electron.
Delving into the Plum Pudding Model
Thomson, the discoverer of the electron, envisioned the atom as a sphere of uniform positive charge. Within this sphere, negatively charged electrons were embedded, much like plums in a pudding or raisins in a cake.
The overall charge of the atom was considered neutral, with the negative charges of the electrons balancing the positive charge of the sphere. It was a simple, elegant model that attempted to reconcile the existence of subatomic particles with the known properties of atoms.
Limitations of the Plum Pudding Model
Despite its initial appeal, the plum pudding model suffered from critical shortcomings. It lacked experimental support and failed to adequately explain several observed phenomena.
One major issue was its inability to account for the scattering of alpha particles by atoms. According to Thomson’s model, alpha particles, being relatively massive and positively charged, should have passed through the diffuse positive charge of the atom with minimal deflection.
The model couldn’t predict or explain the large-angle scattering of alpha particles, an observation that would later become central to Rutherford’s discoveries.
A Model in Need of Replacement
The plum pudding model also struggled to explain the discrete spectral lines emitted by excited atoms. These spectral lines suggested that electrons occupied specific energy levels within the atom, a concept that was entirely absent from Thomson’s model.
The limitations of the plum pudding model highlighted the necessity for a more accurate and comprehensive description of atomic structure. The scientific community was actively seeking an alternative model that could better account for the growing body of experimental evidence.
The stage was set for a revolution in atomic physics, and Ernest Rutherford was poised to deliver it.
The model couldn’t predict or explain the large-angle scattering of alpha particles, an observation that would later become the cornerstone of a revolutionary shift in our understanding of the atom. The stage was set for a confrontation between theory and experimental reality, a clash that would ultimately reshape the landscape of atomic physics.
Surprising Observations and Revolutionary Interpretations
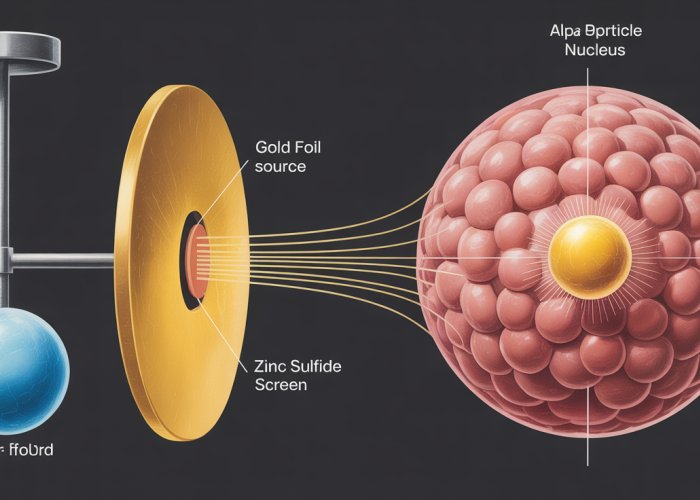
The Gold Foil Experiment, conducted by Geiger and Marsden under Rutherford’s guidance, was designed to validate Thomson’s plum pudding model. The expectation was clear: alpha particles, directed at a thin gold foil, should pass through with minimal deflection, a gentle nudge at most.
Unveiling the Unexpected: Experimental Results
The actual results, however, painted a dramatically different picture. The observations defied expectations, forcing a complete re-evaluation of the prevailing atomic model.
-
The Predominance of Undeflected Particles: The vast majority of alpha particles sailed through the gold foil as if it were empty space. This seemed consistent with the idea of a diffuse positive charge, but the story was far from complete.
-
The Enigma of Large-Angle Deflections: A small, but significant, fraction of alpha particles experienced substantial deflections, veering off course at angles far greater than predicted by the plum pudding model. This was the first crack in the armor of the existing theory.
-
The Astonishing Rebound: Most shockingly, a tiny fraction of alpha particles – approximately 1 in 20,000 – bounced almost directly backward. Rutherford himself described this as "quite the most incredible event that has ever happened to me in my life. It was almost as incredible as if you fired a 15-inch shell at a piece of tissue paper and it came back and hit you."
Rutherford’s Interpretation: A New Atomic Architecture
These unexpected results demanded an explanation. Rutherford, a brilliant experimentalist and theorist, rose to the challenge. He meticulously analyzed the data, formulating a bold new model that could account for the observed phenomena.
-
The Atom is Mostly Empty Space: The high proportion of undeflected alpha particles suggested that the atom was not a solid, uniformly charged sphere, but rather mostly empty space. This was a radical departure from the plum pudding model.
-
The Nucleus: A Center of Concentrated Charge: The large-angle deflections and the backward scattering could only be explained by the presence of a tiny, dense, positively charged core within the atom. Rutherford termed this core the nucleus. The concentration of positive charge and the vast majority of the atom’s mass in this small volume was responsible for the powerful electrostatic forces that deflected the alpha particles.
Rutherford’s interpretation was revolutionary. It not only explained the experimental results but also laid the foundation for a new understanding of atomic structure, one that would fundamentally alter the course of physics and chemistry. The seemingly simple Gold Foil Experiment had unlocked one of nature’s deepest secrets.
The near-complete lack of deflection in the vast majority of alpha particles implied an atom that was mostly empty space. The rare, but significant, large-angle deflections suggested a concentrated, powerful force within the atom. This force was clearly capable of repelling the positively charged alpha particles. The backward bounces, though exceedingly rare, were the most telling. They implied a direct collision with something far more massive than the alpha particles themselves. It was time for a new model.
The Rutherford Atomic Model: A New Vision of the Atom
Rutherford’s interpretation of the Gold Foil Experiment’s results wasn’t just a minor tweak to the existing model; it was a complete paradigm shift. He proposed a radically different picture of the atom, one that challenged the very foundation of the "plum pudding" model. The atom, according to Rutherford, was not a diffuse sphere of positive charge with electrons scattered throughout. Instead, it possessed a concentrated, positively charged nucleus at its center, surrounded by orbiting electrons.
Key Features of the Nuclear Model
The Rutherford model, also known as the nuclear model, rested on a few key tenets:
-
The Nucleus: At the heart of the atom lay a tiny, dense nucleus containing virtually all of the atom’s mass and all of its positive charge. This was a monumental departure from Thomson’s model.
-
Empty Space: The vast majority of the atom’s volume was empty space. This explained why most alpha particles passed through the gold foil undeflected.
-
Orbiting Electrons: Negatively charged electrons orbited the nucleus, much like planets orbiting the sun. This countered the positive charge of the nucleus, resulting in a neutral atom.
Overturning the Thomson Model: A Scientific Revolution
The Rutherford model’s significance lay in its ability to explain the puzzling results of the Gold Foil Experiment. The large-angle deflections and backward scattering could only be explained by the presence of a concentrated, positively charged nucleus. The Thomson model simply couldn’t account for these observations.
The model provided a more accurate representation of atomic structure. It replaced the vague, undefined structure of the plum pudding with a precise arrangement of nucleus and electrons. This shift had a profound impact, opening new avenues of research and laying the groundwork for future advancements in atomic physics.
Acknowledging Geiger and Marsden’s Contributions
While Rutherford is rightly credited with developing the nuclear model, it’s important to acknowledge the crucial contributions of his colleagues, Hans Geiger and Ernest Marsden. It was Geiger and Marsden who meticulously conducted the Gold Foil Experiment, collecting the data that would ultimately lead Rutherford to his groundbreaking conclusions. Their experimental skill and dedication were instrumental in this scientific revolution. Their work was the foundation upon which Rutherford built his model.
Successes and Shortcomings of the Rutherford Model
The Rutherford model, while revolutionary, wasn’t a perfect representation of the atom. It brilliantly explained certain experimental observations, but simultaneously raised new questions and exposed its own inherent limitations. Understanding both its triumphs and its shortcomings is crucial to appreciating its place in the development of atomic theory.
The Model’s Triumphs
The Rutherford model achieved considerable success in several key areas, solidifying its position as a significant advancement over previous models.
Explaining Alpha Particle Scattering
The most immediate and compelling success of the Rutherford model was its ability to explain the results of the Gold Foil Experiment itself. The model elegantly accounted for the observed scattering patterns, including the high proportion of undeflected alpha particles and the rare, but significant, large-angle deflections.
The concept of a small, dense, positively charged nucleus made the observed scattering data not just understandable, but quantitatively predictable.
A Framework for Atomic Structure
Beyond explaining the Gold Foil Experiment, the Rutherford model provided a fundamentally new framework for understanding atomic structure. It established the concept of a central nucleus containing most of the atom’s mass and positive charge, surrounded by orbiting electrons.
This framework became the foundation upon which all subsequent atomic models were built.
It allowed scientists to start thinking about the atom in terms of distinct components and their interactions.
Inherent Limitations and Unanswered Questions
Despite its successes, the Rutherford model suffered from significant limitations that ultimately necessitated further refinement. These limitations primarily stemmed from its inability to reconcile classical physics with the observed behavior of atoms.
The Problem of Atomic Stability
One of the most glaring shortcomings of the Rutherford model was its inability to explain atomic stability. According to classical electromagnetism, an electron orbiting a nucleus should continuously radiate energy in the form of electromagnetic waves.
This radiation would cause the electron to lose energy, gradually spiraling into the nucleus and causing the atom to collapse. However, atoms are demonstrably stable and do not spontaneously collapse.
The Rutherford model offered no explanation for this discrepancy, presenting a major challenge to its validity.
Discrete Spectral Lines
Another significant failure of the Rutherford model was its inability to account for the discrete spectral lines observed in the light emitted by excited atoms. When atoms are heated or otherwise excited, they emit light at specific, well-defined wavelengths, creating a unique spectral fingerprint.
Classical physics, as applied in the Rutherford model, would predict a continuous spectrum of emitted radiation, not the discrete lines that are actually observed. This discrepancy indicated that the behavior of electrons within atoms was governed by principles beyond the scope of classical physics.
The sharp, distinct spectral lines pointed to quantized energy levels within the atom, a concept completely absent from Rutherford’s original model. This crucial piece of the puzzle required a new theoretical approach to be fully understood.
The Rutherford Model’s Enduring Legacy
While the Rutherford model was eventually superseded by more sophisticated theories, its impact on the development of atomic physics remains undeniable. It served as a crucial stepping stone, providing a foundation upon which future scientists could build a more complete and accurate picture of the atom.
A Foundation for Future Advancements
The most significant contribution of the Rutherford model was its establishment of the nuclear model of the atom. Prior to Rutherford, the atom was envisioned as a diffuse entity.
The concept of a central, positively charged nucleus, surrounded by orbiting electrons, completely revolutionized this understanding. This new framework provided a testable model.
It enabled scientists to ask more focused questions and design experiments to probe the atom’s structure in greater detail. It opened the door for advancements.
The Manchester Connection
The groundbreaking Gold Foil Experiment and the subsequent formulation of the Rutherford model were products of research conducted at the University of Manchester. This institution became a hub for early atomic physics research.
It fostered a collaborative environment that nurtured scientific innovation. Manchester University’s role in this pivotal discovery is a testament to the importance of supporting fundamental scientific inquiry.
The Bohr Model: A Quantum Leap Forward
One of the most immediate and significant advancements built upon the Rutherford model was the development of the Bohr model. Niels Bohr, working with Rutherford, recognized the limitations of the classical Rutherford model.
These limitations primarily were its inability to explain atomic stability and the discrete spectral lines observed in atomic emissions. The Bohr model, introduced in 1913, incorporated quantum mechanics.
It postulated that electrons could only occupy specific energy levels or orbits around the nucleus. This quantization of electron energy successfully explained the observed spectral lines of hydrogen and addressed the issue of atomic stability.
While the Bohr model itself was eventually superseded by more advanced quantum mechanical models, it represented a crucial refinement of the Rutherford model. It further solidified the nuclear model.
It demonstrated the power of combining experimental observations with theoretical insights to advance our understanding of the atom. The Rutherford model, therefore, stands as a testament to the power of scientific inquiry.
It is a pivotal moment in the history of physics. It set the stage for the quantum revolution that would reshape our understanding of the universe.
FAQs: Understanding Rutherford’s Atomic Model
Here are some frequently asked questions to help you further understand the Rutherford atomic model and its significance in the development of atomic theory.
What was the main conclusion of Rutherford’s gold foil experiment?
Rutherford’s gold foil experiment demonstrated that most of the atom’s mass and all of its positive charge are concentrated in a very small, dense core called the nucleus. This was a radical departure from the previously held "plum pudding" model.
How did Rutherford’s model differ from the plum pudding model?
The plum pudding model proposed that the atom was a sphere of positive charge with electrons scattered throughout, like plums in a pudding. The rutherford atomic model, however, posited a small, positive nucleus surrounded by mostly empty space where electrons orbited.
What were some limitations of the initial rutherford atomic model?
Rutherford’s initial rutherford atomic model did not explain the stability of the atom. According to classical physics, orbiting electrons should continuously radiate energy and eventually spiral into the nucleus. It also didn’t explain the discrete spectral lines observed in atomic emissions.
Why is the rutherford atomic model considered so important?
Despite its limitations, the rutherford atomic model was a crucial stepping stone in understanding atomic structure. It correctly identified the existence of a nucleus and the separation of positive and negative charges within the atom, paving the way for Bohr’s and subsequent quantum mechanical models.
So, there you have it – a closer look at the rutherford atomic model! Hopefully, this helped clarify some of the key ideas. Now go forth and contemplate the awesome tiny universe within the atom!