Understanding acidity and alkalinity is crucial in various scientific fields. Litmus paper, a common indicator, provides a basic assessment, but the pH colour scale offers a more precise visual representation. The pH meter, a tool often employed by researchers at institutions like the Environmental Protection Agency (EPA), precisely measures the pH of a solution. Interpreting the pH colour scale enables accurate determination of a substance’s pH level.
Unveiling the Secrets of the pH Color Scale
Have you ever wondered why your hydrangeas change color depending on the soil they’re planted in, or why that homemade tomato sauce sometimes tastes a little too acidic? The answer, in many cases, lies in understanding pH. This seemingly simple measurement holds the key to a vast array of chemical reactions and processes that govern our world.
From the health of our ecosystems to the efficiency of industrial processes, pH plays a critical, yet often unseen, role.
The Importance of pH: A World of Applications
Understanding pH isn’t just for scientists in white coats; it’s a fundamental concept that touches nearly every aspect of our lives. Whether you’re a gardener carefully tending to your plants, a chef crafting the perfect dish, or a concerned citizen monitoring water quality, knowledge of pH can empower you to make informed decisions.
But what exactly is pH, and how can we measure it? This blog aims to demystify one of the most accessible tools for pH determination: the pH color scale.
We will explore how the color of a solution, when treated with an indicator, can reveal its acidity or alkalinity.
Decoding the Language of Color
The purpose of this discussion is to unravel the intricacies of the pH color scale, making it a practical and understandable tool for anyone interested in exploring the world of chemistry.
We will delve into the science behind how indicators work. We will show how they change color in response to different pH levels.
We’ll also examine the practical applications of this visual guide across diverse fields, highlighting its utility in:
- Environmental monitoring
- Agriculture
- Chemical research
By the end, you’ll gain a solid understanding of how to interpret the pH color scale and appreciate its significance in a wide range of applications.
But before we plunge into the vibrant spectrum of the pH color scale, it’s essential to build a firm foundation in the underlying concept: pH itself. Grasping the fundamentals of what pH represents and how it’s measured is paramount to truly understanding the information that a color indicator provides.
Deciphering pH: A Fundamental Concept
At its core, pH stands for "Potential of Hydrogen."
This seemingly simple term encapsulates a wealth of chemical information. It represents the concentration of hydrogen ions (H+) in a solution.
Essentially, pH is a measure of the relative acidity or alkalinity of a substance. It allows us to quantify whether a solution leans towards being acidic, basic (alkaline), or neutral.
The pH Scale: A Numerical Spectrum
The pH scale provides a convenient and standardized way to express the acidity or alkalinity of a solution.
It ranges from 0 to 14, with each number representing a specific level of acidity or alkalinity.
A pH of 7 is considered neutral. Pure water, for instance, has a pH very close to 7.
Values below 7 indicate acidity, with lower numbers representing stronger acids.
Lemon juice, with a pH around 2, is a prime example of an acidic substance.
Conversely, values above 7 indicate alkalinity, with higher numbers signifying stronger bases.
Household ammonia, with a pH around 11, demonstrates alkaline properties.
Chemical Properties and pH Values
The pH value of a substance is intimately linked to its chemical behavior and reactivity.
Acids, characterized by a high concentration of hydrogen ions, tend to donate protons in chemical reactions. They often taste sour and can corrode certain materials.
Bases, on the other hand, accept protons and often feel slippery. Strong bases can be caustic and cause severe burns.
The pH of a solution directly influences many chemical reactions, affecting reaction rates, equilibrium positions, and the solubility of various compounds.
Understanding the pH of a substance, therefore, offers crucial insights into its chemical properties and potential interactions. This is key to unlocking various applications in many scientific fields.
Of course. Here is the expanded outline section, formatted for publication as an analytical editorial:
The Science Behind Color Indication: How Indicators Work
Having established a solid understanding of pH and its numerical representation, we can now explore the fascinating world of color indication. This is where chemistry meets visual perception, giving us a practical way to "see" pH levels.
Unveiling pH Indicators: The Color-Changing Chemists
At the heart of the pH color scale lies the concept of pH indicators. These remarkable substances possess the ability to change color in response to alterations in the acidity or alkalinity of a solution.
Think of them as chemical chameleons, shifting their hues to reveal the hidden pH landscape. They are typically weak acids or bases themselves.
Their color-changing behavior is not arbitrary; it’s rooted in intricate chemical reactions.
Universal Indicators: A Spectrum of Possibilities
While some indicators are designed to signal changes within a narrow pH range, universal indicators offer a broader perspective. These are clever mixtures of several different indicators.
This carefully calibrated blend allows them to display a continuous spectrum of colors across a wide pH range (typically from pH 1 to pH 14). This provides a more comprehensive assessment of a solution’s acidity or alkalinity.
By observing the specific color produced by a universal indicator in a solution, one can estimate the pH with reasonable accuracy. Each color corresponds to a specific pH value or a narrow range of values.
The Chemistry of Color Change: A Deeper Dive
So, what’s the underlying chemical mechanism that drives these vibrant color transformations? The answer lies in the molecular structure of the indicator itself.
Equilibrium Shifts and Structural Changes
Indicators are weak acids or bases that exist in equilibrium between two forms: their acidic form (HIn) and their conjugate base form (In-). Each form exhibits a distinct color.
The equilibrium between these two forms shifts depending on the hydrogen ion concentration (H+) in the solution.
In acidic conditions (high H+ concentration), the equilibrium shifts toward the acidic form (HIn), favoring its characteristic color. Conversely, in basic conditions (low H+ concentration), the equilibrium shifts toward the conjugate base form (In-), resulting in its distinct color.
Absorption of Light and Perceived Color
The color we perceive is determined by the wavelengths of light that a substance absorbs and reflects.
The acidic and basic forms of an indicator have different molecular structures, which means they absorb different wavelengths of light.
For example, if a substance absorbs blue and green light, it will appear red to our eyes (because red light is being reflected).
As the pH of the solution changes, the relative amounts of the acidic and basic forms of the indicator shift. This leads to a change in the wavelengths of light absorbed and reflected, thus changing the observed color.
This interplay between chemical equilibrium, molecular structure, and light absorption is what makes pH indicators such powerful tools for visualizing acidity and alkalinity.
Of course. Here is the expanded outline section, formatted for publication as an analytical editorial:
Decoding the pH Color Scale: A Visual Guide
Having established a solid understanding of pH and its numerical representation, we can now explore the fascinating world of color indication. This is where chemistry meets visual perception, giving us a practical way to "see" pH levels.
The pH Rainbow: Linking Color to Acidity
The pH color scale is essentially a visual translation of the pH values we discussed earlier. It provides an intuitive way to estimate the acidity or alkalinity of a solution based on its observed color when a pH indicator is present.
Understanding this color-coding system is crucial for anyone working with acids and bases.
Color Ranges and pH Values: A Detailed Breakdown
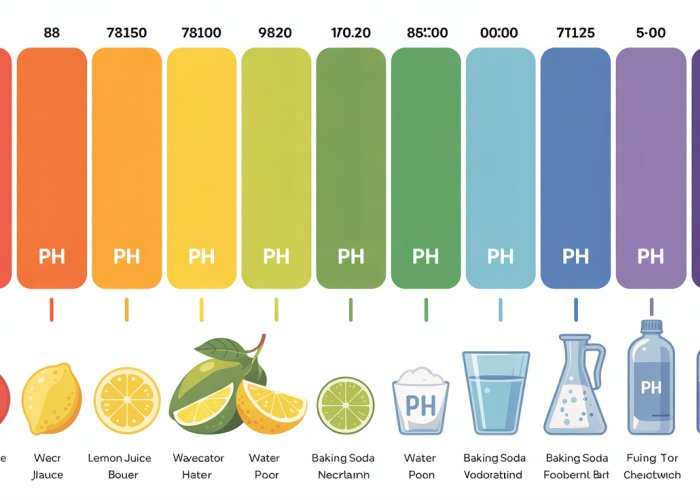
Let’s break down the typical color ranges associated with different pH levels. Keep in mind that these are general guidelines, and the exact colors can vary slightly depending on the specific indicator used.
-
Strong Acids (pH 1-3): Solutions in this range typically display vibrant red or orange hues. The intensity of the red often correlates with the strength of the acid. A deep red indicates a highly concentrated, strongly acidic solution.
-
Weak Acids (pH 4-6): As the pH increases and we move into the realm of weaker acids, the colors transition towards yellow. A pale yellow or yellowish-orange might indicate a weakly acidic solution.
-
Neutral Substances (pH 7): Neutral substances, like pure water, ideally exhibit a green color. This green serves as a visual benchmark, indicating neither acidic nor basic properties.
-
Weak Bases (pH 8-10): On the alkaline side of the spectrum, colors shift towards blue. A light blue or greenish-blue suggests a weakly basic solution.
-
Strong Bases (pH 11-14): Strong bases are characterized by deep blue or violet colors. Similar to strong acids, the intensity of the blue often reflects the concentration or strength of the base. A deep violet signifies a highly alkaline solution.
Color Intensity: A Qualitative Indicator of Strength
It’s important to note that color intensity can provide a qualitative indication of the strength of the acid or base.
While not a precise measurement, a more intense color generally suggests a higher concentration of acid or base. This is a useful rule of thumb, but it should be used cautiously and in conjunction with other observations.
Visual Aids: Charts and Color Guides
Many resources offer visual aids, such as pH color charts, that display a spectrum of colors corresponding to different pH values.
These charts can be invaluable tools for comparing observed colors and estimating pH levels. When using these charts, it’s important to ensure that the lighting conditions are consistent, as lighting can significantly affect color perception.
Having explored how color visually represents acidity and alkalinity, it’s time to examine some of the common tools that bring this color-coding to life. Certain substances, known as indicators, possess the remarkable ability to change color in response to varying pH levels. Let’s delve into these everyday workhorses of acid-base chemistry.
Common Indicators: Litmus, Universal, and Beyond
Litmus Paper: The Classic Acid-Base Test
Litmus paper is perhaps the most widely recognized pH indicator. This simple tool, often used in introductory chemistry, consists of paper treated with a natural dye extracted from lichens.
Its primary function is to distinguish between acidic and basic solutions.
Litmus paper comes in two forms: red litmus paper and blue litmus paper.
- Red litmus paper turns blue in alkaline (basic) solutions.
- Blue litmus paper turns red in acidic solutions.
It’s important to note that litmus paper only indicates whether a solution is generally acidic or basic; it does not provide a precise pH value. Its simplicity and ease of use make it a valuable tool for quick, qualitative assessments.
Limitations of Litmus Paper
While incredibly convenient, litmus paper has limitations. It cannot discern the strength of an acid or base, nor can it pinpoint the exact pH. A strong acid and a weak acid will both turn blue litmus red, even though their pH values differ significantly.
Universal Indicator Solutions: A Broader Spectrum
For a more detailed indication of pH, universal indicator solutions are employed. These solutions are mixtures of several different indicators, carefully selected to exhibit a range of color changes across the entire pH scale (typically 1-14).
Universal indicators provide a spectrum of colors, each corresponding to a specific pH range. By comparing the color of a solution with a color chart provided with the indicator, one can estimate the pH value with reasonable accuracy.
Universal Indicator Color Chart
Typically, a universal indicator color chart will show a progression of colors, something like this:
- Strongly Acidic (pH 1-3): Red
- Moderately Acidic (pH 4-6): Orange to Yellow
- Neutral (pH 7): Green
- Moderately Alkaline (pH 8-10): Blue
- Strongly Alkaline (pH 11-14): Violet to Purple
The exact colors can vary slightly depending on the specific formulation of the universal indicator, so always refer to the manufacturer’s chart.
Beyond Litmus and Universal Indicators
While litmus paper and universal indicators are staples, a vast array of other pH indicators exists, each with its own specific pH range and color transition. These specialized indicators are often used in titrations and other analytical techniques where precise pH determination is crucial.
Methyl Orange and Phenolphthalein
Two examples are methyl orange and phenolphthalein. Methyl orange changes color in the acidic range (pH 3.1-4.4), transitioning from red to yellow. This makes it suitable for titrations involving strong acids.
Phenolphthalein, on the other hand, is colorless in acidic solutions and turns pink to magenta in alkaline solutions (pH 8.3-10.0). It is commonly used in titrations involving weak acids and strong bases.
Tailoring the Choice to the Application
The choice of indicator depends heavily on the specific application. For simple acid-base identification, litmus paper suffices. When a more precise pH estimate is needed, universal indicators are preferred. For highly accurate pH determination in titrations or other analytical procedures, specific indicators with narrow transition ranges are selected.
Universal indicator solutions offer a more granular view, boasting a spectrum of colors tied to specific pH values. But even with these advancements, achieving accuracy with color-based pH determination isn’t always straightforward. Several factors can subtly, or not so subtly, skew results.
Factors Influencing the pH Color Scale: Considerations for Accuracy
The beauty of using a color-based pH scale lies in its relative simplicity and visual nature. However, it’s crucial to acknowledge that several factors can significantly influence the accuracy of pH measurements obtained this way. Understanding these influences allows for more informed interpretations and minimizes potential errors.
Temperature’s Impact on pH Measurement
Temperature plays a crucial role in chemical equilibria, and acid-base reactions are no exception. The pH of a solution is inherently temperature-dependent. As temperature changes, the dissociation constants of acids and bases shift, altering the equilibrium concentrations of H+ and OH- ions.
This shift directly affects the observed pH value.
For example, the pH of pure water is precisely 7 at 25°C. However, at higher temperatures, water becomes slightly more acidic (pH < 7), while at lower temperatures, it becomes slightly more alkaline (pH > 7).
This effect is more pronounced in solutions containing weak acids or bases. Therefore, it is essential to control or at least note the temperature at which pH measurements are taken and, if possible, calibrate indicators or pH meters at the same temperature.
Indicator Concentration and Color Perception
The concentration of the indicator solution used can also influence the observed color and, consequently, the estimated pH. If the indicator concentration is too high, the solution’s color may be overly intense, masking subtle shifts in hue that reflect precise pH changes.
This can lead to inaccurate readings, especially near transition points where the color change is most gradual. Conversely, too low of an indicator concentration may produce a faint color, making it difficult to discern the precise shade and again leading to inaccuracies.
Furthermore, the human eye’s perception of color is subjective and can be affected by ambient lighting conditions. Always compare the indicator color to a standardized color chart under consistent lighting to minimize subjective errors.
Interferences from Other Substances
The presence of other substances in the solution being tested can interfere with the indicator’s color change, leading to inaccurate pH readings. Certain ions or molecules may react with the indicator itself, preventing it from exhibiting its characteristic color at a given pH.
For instance, strongly colored solutions can mask the indicator’s color, making it difficult to determine the pH accurately. Similarly, oxidizing or reducing agents can alter the chemical structure of the indicator, leading to unexpected color changes.
Turbidity or the presence of suspended particles can scatter light, further complicating color interpretation. In complex samples, such as soil or biological fluids, these interferences are common and must be considered.
Whenever possible, it’s crucial to pre-treat samples to remove potential interfering substances before adding the indicator. This might involve filtration, dilution, or the addition of masking agents that selectively bind to interfering ions.
Factors such as temperature and indicator concentration can introduce variability into pH measurements derived from color scales. Even with careful technique, relying solely on color can present limitations. But where does this seemingly simple yet nuanced method of pH determination find its purpose in the real world?
Real-World Applications: Where the pH Color Scale Matters
The pH color scale, despite its inherent limitations, plays a vital role across a surprising range of disciplines. Its portability, ease of use, and relatively low cost make it an indispensable tool in situations where rapid, approximate pH measurements are needed. Let’s explore some key areas where this method shines.
Environmental Monitoring: Safeguarding Our Ecosystems
Assessing Water Quality
The health of aquatic ecosystems is profoundly affected by pH. Deviations from the optimal pH range can harm or eliminate sensitive species. The pH color scale offers a quick and accessible way to assess water quality in rivers, lakes, and streams.
Environmental scientists and conservationists frequently use indicator solutions to perform on-site pH tests, helping to identify potential pollution sources or the impacts of acid rain.
Detecting and Mitigating Acid Rain
Acid rain, caused by atmospheric pollutants, lowers the pH of precipitation and can damage forests, soils, and aquatic life. Monitoring rainfall pH with indicators provides crucial data for tracking the extent and severity of acid rain.
This information is vital for implementing strategies to reduce emissions and mitigate the environmental impact of industrial activities.
Agriculture: Cultivating Optimal Growth Conditions
Soil pH and Nutrient Availability
Soil pH is a critical factor influencing nutrient availability for plants. Different plant species thrive within specific pH ranges. Measuring soil pH using color indicators allows farmers and gardeners to determine whether their soil is suitable for particular crops.
If the pH is too high or too low, they can take corrective measures, such as adding lime to raise pH or sulfur to lower it, ensuring optimal nutrient uptake and healthy plant growth.
Optimizing Fertilizer Use
Understanding soil pH also helps optimize fertilizer use. Fertilizers are most effective when the soil pH is within the appropriate range, allowing plants to efficiently absorb the nutrients they need. By monitoring soil pH, farmers can reduce fertilizer waste, minimize environmental pollution, and maximize crop yields.
Chemical Laboratories: A Versatile Analytical Tool
Acid-Base Titrations
While pH meters offer greater precision, color indicators remain a valuable tool in chemical laboratories, particularly for acid-base titrations. Indicators signal the endpoint of a titration reaction through a distinct color change, allowing chemists to determine the concentration of an unknown acid or base.
Qualitative Analysis
Indicators are also useful for quick qualitative analysis of samples. They can provide a rapid indication of whether a solution is acidic, basic, or neutral, guiding further experimentation and analysis.
Everyday Life: pH at Home and Beyond
Swimming Pool Maintenance
Maintaining the correct pH balance in swimming pools is essential for sanitation and swimmer comfort. Pool test kits that utilize color indicators are readily available, enabling pool owners to easily monitor and adjust pH levels, ensuring a safe and enjoyable swimming experience.
Food pH and Preservation
The pH of food affects its taste, texture, and preservation. Color indicators can be used to assess the acidity of homemade pickles, jams, and other preserved foods. Proper pH levels are crucial for inhibiting the growth of harmful bacteria and ensuring food safety.
Advantages and Limitations: Weighing the Pros and Cons
Like any analytical tool, the pH color scale comes with its own set of strengths and weaknesses. While it offers a convenient way to estimate pH, it’s crucial to understand both its advantages and limitations to ensure its appropriate use and accurate interpretation of results.
The Allure of Simplicity: Advantages of the pH Color Scale
The pH color scale’s enduring popularity stems from its simplicity and practicality. It provides a rapid, straightforward method for estimating pH, making it accessible to users with varying levels of scientific expertise.
Speed and Convenience
One of the most significant advantages of using a pH color scale is the speed at which a measurement can be obtained.
Unlike electronic pH meters that require calibration and setup, color indicators provide an almost instantaneous result. Simply add a few drops of the indicator solution to the sample or dip the indicator paper, and observe the resulting color change.
User-Friendliness
The pH color scale is remarkably easy to use, requiring minimal training or specialized knowledge.
This makes it ideal for field applications, educational settings, and situations where technical expertise may be limited.
Cost-Effectiveness
Compared to electronic pH meters, which can be expensive to purchase and maintain, pH indicators are highly cost-effective.
This makes them a practical option for applications where budgetary constraints are a concern. Indicators are especially useful when multiple, simultaneous pH measurements are needed across a wide area.
The Shadows of Subjectivity: Limitations of the pH Color Scale
Despite its advantages, the pH color scale is not without its limitations. It is essential to recognize these limitations to avoid misinterpretations and ensure the validity of the results.
Subjectivity in Color Interpretation
The primary limitation of the pH color scale is the subjectivity involved in interpreting color changes.
Human perception of color can vary due to individual differences in color vision, lighting conditions, and the concentration of the indicator used.
This subjectivity can lead to inconsistencies and inaccuracies in pH estimation.
Limited Accuracy Compared to pH Meters
While the pH color scale can provide a reasonable estimate of pH, it is less accurate than electronic pH meters.
pH meters provide a digital readout of the pH value, offering greater precision and minimizing the potential for human error.
Potential for Color Interference
The presence of other colored substances in the sample can interfere with the color interpretation of the pH indicator.
This is especially problematic in complex samples such as soil extracts or industrial effluents.
The interfering colors can mask or distort the indicator’s color change, leading to inaccurate pH estimations.
FAQs About the pH Color Scale
Here are some frequently asked questions about the pH color scale to help you better understand how it works and its applications.
How does the pH color scale work?
The pH color scale indicates the acidity or alkalinity of a substance. A chemical indicator changes color depending on the pH level. The resulting color is then matched to a standard color chart, revealing the pH.
What pH values correspond to acidic, neutral, and alkaline solutions?
On the pH color scale, values from 0 to 6 are acidic, with 0 being the most acidic. A pH of 7 is neutral. Values from 8 to 14 are alkaline (or basic), with 14 being the most alkaline.
Can the pH color scale be used for any liquid?
While the pH color scale is versatile, its accuracy can be affected by factors like the presence of strong oxidizers or reducers. For precise measurements, especially in complex solutions, a pH meter is generally preferred over relying solely on the pH color scale.
What are some common uses for pH indicators and the pH color scale?
pH indicators and the pH color scale are widely used in education, basic lab experiments, soil testing for gardening, and monitoring water quality. They provide a quick and easy way to estimate pH without requiring expensive equipment.
So, there you have it! Hopefully, you now have a much clearer understanding of the pH colour scale. Go forth and experiment responsibly. Until next time!