The phenomenon of selective passage, central to biological processes, directly influences the complexity surrounding questions on osmosis. Understanding cell membrane permeability, a core principle of osmosis, enhances insight into the mechanism answering the common questions on osmosis. Tonicity, a defining property of solutions explored in the experiments performed at the University of California, is crucial for addressing practical questions on osmosis that arise. Osmotic pressure, measured using tools such as the osmometer, reveals the fundamental forces underpinning questions on osmosis in diverse systems.
Crafting the Ultimate Guide: "Osmosis Questions Answered"
Our aim is to create a comprehensive resource addressing "questions on osmosis." The article needs a logical flow that starts with the basics and progressively moves towards more complex applications and nuances. Clarity and accessibility are paramount.
1. Introduction: What is Osmosis and Why Should You Care?
This section serves as an entry point for readers with varying levels of prior knowledge.
- Hook: Begin with a relatable scenario or a surprising fact about osmosis’s role in everyday life (e.g., how plants absorb water, why salted meat lasts longer).
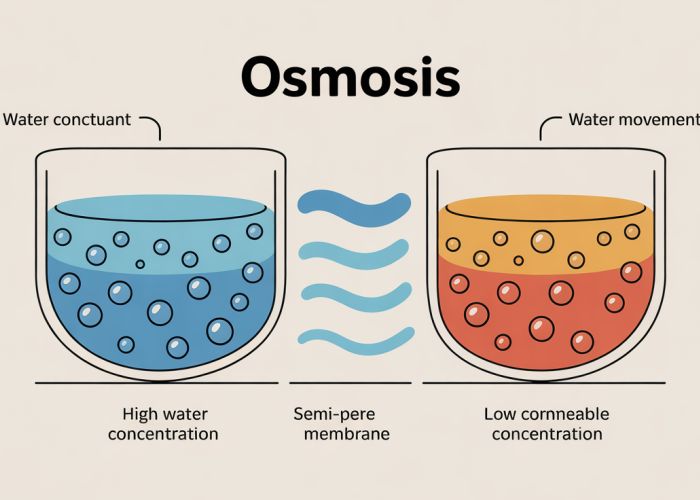
- Definition: Provide a clear and concise definition of osmosis. Emphasize it’s a specific type of diffusion. Explain the movement of solvent (usually water) across a semipermeable membrane.
- Significance: Briefly highlight the importance of osmosis in various fields like biology, chemistry, and even cooking. This answers the “Why should I care?” question upfront.
- Keyword Integration: Naturally incorporate "questions on osmosis" within the introduction. For example: "Many people have questions on osmosis related to how it works and its real-world implications. This guide aims to answer those questions on osmosis in detail."
2. The Fundamentals of Osmosis: Understanding the Basics
This section delves into the core principles governing osmosis.
2.1 Defining Key Terms: A Glossary
A table or bulleted list defining essential terms is crucial for readers to follow along.
- Solvent: (usually water).
- Solute: (e.g., salt, sugar).
- Solution: (mixture of solvent and solute).
- Semipermeable Membrane: (membrane that allows some molecules to pass through but not others).
- Concentration Gradient: (difference in concentration of a substance between two areas).
- Osmotic Pressure: (pressure required to prevent osmosis from occurring).
2.2 The Mechanism Explained: How Osmosis Works
- Step-by-step Explanation: Describe the process of osmosis in a clear, step-by-step manner. Use visual aids like diagrams if possible.
- Focus on Water Movement: Emphasize that water moves from an area of high water concentration (low solute concentration) to an area of low water concentration (high solute concentration).
- Address Misconceptions: Briefly address common misconceptions about osmosis.
2.3 Factors Affecting Osmosis: What Influences the Rate?
- Concentration Gradient: Explain how a steeper concentration gradient leads to a faster rate of osmosis.
- Temperature: Discuss the effect of temperature on the kinetic energy of molecules and how this impacts the rate of osmosis.
- Pressure: Explain how external pressure can affect the direction and rate of osmosis.
- Membrane Permeability: Discuss how the characteristics of the semipermeable membrane (e.g., pore size) influence the rate of osmosis.
3. Answering Common Questions on Osmosis
This is the core of the article, directly addressing "questions on osmosis."
3.1 Basic Conceptual Questions
Use a Q&A format to address common conceptual "questions on osmosis."
- Q: Why does osmosis occur? A: Osmosis occurs to equalize the concentration of solute on both sides of a semipermeable membrane.
- Q: Does osmosis require energy? A: No, osmosis is a passive process.
- Q: What happens when equilibrium is reached during osmosis? A: The net movement of water stops, but water molecules still move across the membrane at equal rates in both directions.
- Q: Can osmosis occur with solutions other than water? A: Yes, but water is the most common solvent in biological systems.
3.2 Questions About Osmotic Pressure
- Q: What is osmotic pressure, and how is it measured? A: Provide a detailed explanation of osmotic pressure and mention methods for measuring it.
- Q: How does osmotic pressure relate to concentration? A: Explain the direct relationship between solute concentration and osmotic pressure. Use relevant formulas if appropriate.
- Q: What happens if the external pressure exceeds the osmotic pressure? A: Explain reverse osmosis in this context.
3.3 Questions Related to Living Organisms
- Q: How does osmosis help plants absorb water? A: Explain the role of osmosis in root hair cells.
- Q: What happens to animal cells in a hypotonic, isotonic, and hypertonic solution? A: Use diagrams to illustrate cell behavior in different solutions (lysing, normal, crenation).
- Q: How does osmosis maintain cell turgor pressure in plants? A: Explain the importance of turgor pressure for plant rigidity.
- Q: How does the body regulate osmotic pressure (osmoregulation)? A: Briefly touch upon osmoregulation in animals.
3.4 Advanced and Less Common Questions
This section can address more nuanced "questions on osmosis."
- Q: What is reverse osmosis, and how is it used in water purification? A: Explain the process of reverse osmosis and its applications.
- Q: What is the Van ‘t Hoff factor and how does it affect osmotic pressure calculations? A: Discuss the Van ‘t Hoff factor for ionic compounds.
- Q: How is osmosis utilized in various industrial processes beyond water purification? A: Briefly describe other applications.
4. Real-World Applications of Osmosis
This section showcases the practical uses of osmosis, making the topic more engaging.
- Food Preservation: Explain how salt and sugar act as preservatives by drawing water out of bacteria through osmosis.
- Medical Applications: Discuss the use of isotonic solutions in IV fluids and the importance of osmosis in kidney function.
- Water Purification: Elaborate on the use of reverse osmosis in desalinating seawater and producing potable water.
- Agriculture: Discuss the importance of osmotic potential in plant growth and how farmers manage soil salinity.
5. Troubleshooting and Potential Issues
This section addresses potential problems and offers solutions.
- Common Experimental Errors: Discuss potential errors when conducting osmosis experiments (e.g., membrane leaks, inaccurate measurements).
- Troubleshooting Slow or No Osmosis: Provide tips for identifying and resolving issues that can prevent osmosis from occurring.
- Interpreting Results: Offer guidance on how to interpret data obtained from osmosis experiments.
6. Further Exploration
This section provides avenues for readers to delve deeper into the topic.
- Suggested Reading: List relevant textbooks, scientific articles, and reputable websites.
- Online Resources: Provide links to interactive simulations, videos, and online courses.
- Experiment Ideas: Suggest simple osmosis experiments that readers can conduct at home or in a classroom setting.
Osmosis: Frequently Asked Questions
We’ve compiled some common questions on osmosis to help clarify concepts from "Osmosis Questions Answered: The Ultimate Guide!".
What exactly is osmosis?
Osmosis is the movement of water across a semipermeable membrane from an area of high water concentration to an area of low water concentration. This process aims to equalize the concentration of solutes on both sides of the membrane. Understanding this definition is key when you have more questions on osmosis.
How is osmosis different from diffusion?
While both osmosis and diffusion involve the movement of molecules from areas of high concentration to low concentration, there’s a key difference. Diffusion can involve any type of molecule, while osmosis specifically refers to the movement of water across a semipermeable membrane. You might have more questions on osmosis as it is a type of diffusion.
What are some real-world examples of osmosis?
Osmosis is vital in many biological processes. For example, plant roots absorb water from the soil through osmosis. It’s also crucial for maintaining cell turgor pressure and the absorption of nutrients in the small intestine. You will encounter questions on osmosis in studying of biological process.
What happens to a cell if it’s placed in a hypertonic solution?
In a hypertonic solution, the concentration of solutes outside the cell is higher than inside. Water will move out of the cell via osmosis to try and balance the solute concentration, causing the cell to shrink. Understanding this effect is crucial when considering further questions on osmosis.
So, did this clear up your questions on osmosis? Hopefully, you’ve got a much better grasp now. If you’re still scratching your head, don’t worry – keep exploring, and those ‘aha!’ moments will come. Good luck with your studies!