Understanding the polarity of molecules is fundamental in chemistry, particularly when analyzing hydrocarbons like octane. Molecular geometry, a key concept studied at institutions such as MIT, significantly influences whether a compound exhibits polar or nonpolar characteristics. The dipole moment, often measured using specialized instruments, provides quantitative data that helps determine the overall polarity. So, based on the principles of chemical bonding and molecular structure, is octane polar, or does its structure make it a nonpolar compound?
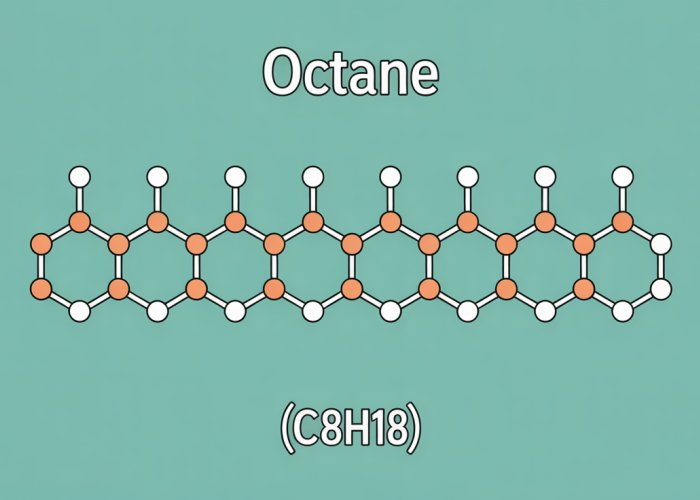
Octane, a ubiquitous hydrocarbon with the chemical formula C8H18, is a major constituent of gasoline, the fuel that powers a significant portion of the world’s transportation.
Its presence is so deeply ingrained in our daily lives that we often take its properties for granted.
However, understanding octane’s fundamental characteristics, such as its polarity, is crucial for grasping its behavior in various chemical and physical contexts.
The Central Question: Polar or Nonpolar?
The fundamental question we aim to address is: Is octane a polar or nonpolar molecule?
This seemingly simple inquiry unlocks a deeper understanding of octane’s interactions with other substances, its physical properties (like boiling point), and its role in combustion processes.
To answer this, we must explore the intricacies of its molecular structure and the nature of chemical bonds.
Molecular Polarity: Predicting Properties and Behavior
The concept of molecular polarity is not merely an academic exercise; it is a powerful tool for predicting a substance’s properties and behavior.
Polarity dictates how molecules interact with each other, influencing everything from solubility and miscibility to reactivity and biological activity.
For instance, polar molecules tend to dissolve well in polar solvents (like water), while nonpolar molecules favor nonpolar solvents (like oil).
Understanding octane’s polarity allows us to predict its miscibility with other gasoline components, its behavior during combustion, and its potential environmental impact.
Octane’s place in the world hinges on the behavior dictated by its molecular structure. This behavior, how it interacts with other substances, its physical state, and its flammability, is closely connected to the underlying forces that govern molecule interactions. To truly understand octane, it’s vital to first understand polarity, and the factors that give rise to this property.
Understanding Polarity: A Chemical Perspective
At its core, polarity describes the distribution of electrical charge within a molecule. This distribution isn’t always uniform. It can be uneven, leading to regions of partial positive and partial negative charge. This unevenness is what defines a polar molecule.
Defining Polarity in Chemical Bonds
Polarity in a chemical bond arises from the unequal sharing of electrons between two atoms. This happens when one atom has a greater affinity for electrons than the other. This affinity is quantified by a property called electronegativity.
The more electronegative atom pulls the shared electrons closer to itself, resulting in a partial negative charge (δ-) on that atom. Conversely, the less electronegative atom acquires a partial positive charge (δ+). This separation of charge creates a dipole moment, which is a measure of the magnitude and direction of the polarity.
Think of it like a tug-of-war where one side is stronger. The rope (representing the shared electrons) is pulled closer to the stronger side, creating an imbalance.
Characteristics of Nonpolar Molecules
In stark contrast to polar molecules, nonpolar molecules exhibit an equal distribution of charge. This even distribution arises in two primary scenarios.
The first is when two atoms of the same element are bonded together, such as in diatomic molecules like hydrogen (H2) or oxygen (O2). In these cases, both atoms have identical electronegativity, so they share the electrons equally.
The second scenario occurs when different atoms are bonded, but their electronegativity difference is negligible. Furthermore, even if polar bonds are present, a molecule can be nonpolar if its geometry cancels out the individual bond dipoles.
For example, carbon dioxide (CO2) has two polar bonds (C=O), but its linear geometry ensures that the dipole moments of the two bonds cancel each other out, resulting in a nonpolar molecule.
Electronegativity and Bond Polarity
Electronegativity is the key determinant of bond polarity. It is a measure of an atom’s ability to attract electrons in a chemical bond. Elements with high electronegativity, such as fluorine (F) and oxygen (O), strongly attract electrons, while elements with low electronegativity, such as sodium (Na) and potassium (K), weakly attract electrons.
Linus Pauling famously developed the electronegativity scale. Large differences in electronegativity between two bonded atoms lead to polar bonds. A general rule of thumb is that if the electronegativity difference is greater than 0.4, the bond is considered polar. The larger the difference, the more polar the bond.
Understanding polarity opens a window into predicting a molecule’s behavior. To truly grasp why octane behaves the way it does, we must delve into its molecular architecture, meticulously examining how its structure dictates its nonpolar character.
Octane’s Molecular Structure: Geometry and Influence on Polarity
Octane’s nonpolar nature isn’t just a coincidence. It’s a direct consequence of its specific arrangement of atoms in three-dimensional space. To understand this, we need to unpack the elements of its structure.
The Foundation: Chemical Formula and Structure
Octane’s chemical formula, C8H18, immediately tells us that it is composed solely of carbon and hydrogen atoms. This classification as a hydrocarbon is the first major clue to its nonpolar tendencies.
The arrangement of these atoms can take different forms. The most common form is a straight-chain alkane, where the eight carbon atoms are linked in a continuous sequence.
However, octane can also exist as branched isomers, where the carbon chain branches off.
Regardless of whether it is the straight chain or branched isomer, the fundamental structural units remain the same: carbon-carbon and carbon-hydrogen bonds.
Visualizing Octane: Arrangement of Carbon and Hydrogen
Imagine a chain of eight carbon atoms, each linked to its neighbors. The remaining bonds on each carbon atom are saturated with hydrogen atoms. This results in a molecule where carbon forms the backbone and hydrogen atoms surround it.
Each carbon atom in the chain forms four bonds, adhering to the octet rule. The spatial arrangement of these bonds plays a critical role in determining the overall polarity.
Molecular Geometry and its Implications
Each carbon atom in octane exhibits tetrahedral geometry. This means that the four atoms bonded to each carbon atom are arranged in a three-dimensional shape resembling a tetrahedron, with the carbon atom at the center.
This tetrahedral arrangement is crucial because it leads to a symmetrical distribution of the nonpolar C-H bonds around each carbon atom.
Because carbon and hydrogen have relatively similar electronegativities, the C-H bonds are considered essentially nonpolar. The slight difference in electronegativity is not enough to create a significant dipole moment.
The symmetrical arrangement of these nonpolar C-H bonds around each carbon atom effectively cancels out any potential polarity within the molecule.
In essence, the individual bond dipoles, if they existed to any significant degree, would counteract each other due to the tetrahedral geometry. This results in an overall molecule with no net dipole moment, confirming its nonpolar character.
Octane’s nonpolar character stems from its molecular structure, where carbon and hydrogen atoms are linked in a largely symmetrical manner. However, to fully appreciate why octane behaves as it does, it’s essential to place it within the context of its broader chemical family: hydrocarbons.
Octane as a Hydrocarbon: A Class of Nonpolar Compounds
Octane is unequivocally a hydrocarbon, a classification that carries significant implications for its polarity. Hydrocarbons, by definition, are organic compounds composed exclusively of carbon and hydrogen atoms. This seemingly simple composition is the key to understanding their shared properties, particularly their nonpolar nature.
Defining Hydrocarbons
Hydrocarbons are the foundational building blocks of fossil fuels like gasoline, natural gas, and petroleum. Their structures can vary widely, ranging from simple linear chains to complex cyclic or branched arrangements. Despite this structural diversity, the common thread among all hydrocarbons is their elemental makeup: carbon and hydrogen.
The Electronegativity Factor
The similarity in electronegativity between carbon and hydrogen is the primary reason for the nonpolar nature of most hydrocarbons. Electronegativity is a measure of an atom’s ability to attract shared electrons in a chemical bond. Carbon has an electronegativity value of approximately 2.55, while hydrogen’s is around 2.20.
This relatively small difference of 0.35 means that the electrons in a carbon-hydrogen (C-H) bond are shared nearly equally.
Consequently, there is minimal charge separation, and the bond is considered nonpolar.
Implications for Molecular Polarity
While individual C-H bonds may have a slight dipole moment, the overall molecular polarity of a hydrocarbon depends on the arrangement of these bonds. In symmetrical molecules like octane, these small bond dipoles effectively cancel each other out.
This cancellation results in a molecule with a negligible overall dipole moment, thus confirming its nonpolar character.
Even in branched hydrocarbons, the presence of only C-H and C-C bonds (where the electronegativity difference is zero) ensures that the molecule remains largely nonpolar.
Hydrocarbons Beyond Octane
The principle of nonpolarity extends to a wide range of other hydrocarbons, including methane, ethane, propane, and butane. These compounds share the same fundamental characteristics as octane: they are composed of only carbon and hydrogen and exhibit minimal polarity.
Their nonpolar nature dictates many of their physical properties, such as their insolubility in water and their tendency to exist as gases or liquids with low boiling points at room temperature.
Understanding octane as a representative of the hydrocarbon family provides a valuable framework for predicting the behavior and properties of related organic compounds. The unifying feature of carbon and hydrogen composition dictates its nonpolar nature.
Intermolecular Forces in Octane: Weak Attractions
Having established octane’s nonpolar nature, it’s time to explore how this characteristic influences its physical behavior. The key lies in the types of intermolecular forces (IMFs) that govern how octane molecules interact with each other. Understanding these IMFs is crucial for predicting properties such as boiling point and viscosity.
Understanding Intermolecular Forces
Intermolecular forces are the attractive or repulsive forces that exist between molecules. These forces are distinct from intramolecular forces, which are the forces that hold atoms together within a molecule (i.e., chemical bonds). IMFs are generally much weaker than chemical bonds, but they are still responsible for many of the bulk properties of matter.
IMFs play a critical role in determining whether a substance exists as a solid, liquid, or gas at a given temperature. They also influence a substance’s boiling point, melting point, viscosity, and surface tension.
London Dispersion Forces: The Dominant IMF in Octane
Octane, being a nonpolar molecule, primarily experiences London dispersion forces (LDFs), also known as Van der Waals forces. LDFs are temporary, short-range attractions that arise from instantaneous fluctuations in electron distribution within molecules.
Even in nonpolar molecules, electrons are not always evenly distributed. At any given moment, there may be a slight, temporary concentration of electrons on one side of the molecule, creating a transient dipole. This temporary dipole can then induce a similar dipole in a neighboring molecule, leading to a weak attractive force.
The strength of London dispersion forces depends on the size and shape of the molecule. Larger molecules with more electrons tend to have stronger LDFs because there are more opportunities for temporary dipoles to form. Octane, with its eight carbon atoms and eighteen hydrogen atoms, is large enough to exhibit significant, though still relatively weak, LDFs.
Impact on Boiling Point and Physical State
The weakness of London dispersion forces in octane directly explains its relatively low boiling point (approximately 125 °C or 257 °F). Because the attractive forces between octane molecules are weak, less energy is required to overcome these forces and transition octane from a liquid to a gaseous state.
At room temperature (around 25 °C or 77 °F), octane exists as a liquid. The kinetic energy of the molecules is sufficient to overcome the weak LDFs, preventing them from forming a rigid, solid structure. However, the IMFs are strong enough to keep the molecules relatively close together, resulting in the liquid state.
The relatively low viscosity of octane can also be attributed to its weak IMFs. Viscosity is a measure of a fluid’s resistance to flow. Because the octane molecules are not strongly attracted to each other, they can move past one another relatively easily, resulting in a low viscosity compared to substances with stronger intermolecular forces.
Dipole Moment Analysis: Quantifying Octane’s Nonpolarity
The discussion about octane’s nonpolar nature has so far been qualitative, based on molecular structure and the electronegativity differences between carbon and hydrogen. Now, we turn to a more quantitative measure to solidify our understanding: the dipole moment.
Introducing Dipole Moment
The dipole moment is a vector quantity that describes the separation of positive and negative charges in a molecule. It’s essentially a measure of the molecule’s polarity.
A molecule with a significant dipole moment is considered polar, while a molecule with a dipole moment close to zero is considered nonpolar.
The dipole moment is typically measured in Debye units (D).
It’s important to remember that even if individual bonds within a molecule are polar, the overall molecule may be nonpolar if the bond dipoles cancel each other out due to symmetry.
Octane’s Negligible Dipole Moment
Octane, as we’ve established, is a nonpolar molecule. This is reflected in its exceedingly small, practically negligible, dipole moment.
While individual C-H bonds do possess a slight polarity due to the small electronegativity difference between carbon and hydrogen, the highly symmetrical structure of octane is the key factor.
Consider a straight-chain octane molecule. The C-H bond dipoles are oriented in such a way that they effectively cancel each other out, resulting in a net dipole moment that is very close to zero.
Even branched isomers of octane, while potentially having slightly higher dipole moments than the straight-chain isomer, still exhibit very low values that are characteristic of nonpolar substances.
Therefore, the near-zero dipole moment of octane strongly supports its classification as a nonpolar compound.
Experimental Verification and Theoretical Predictions
While we can predict octane’s nonpolarity based on its structure and electronegativity considerations, experimental measurements of its dipole moment provide crucial validation.
Scientists can use techniques like dielectric constant measurements to experimentally determine the dipole moment of a substance.
These experimental results consistently show that octane has a very low dipole moment, typically on the order of 0.0 – 0.1 Debye, essentially confirming the theoretical predictions.
Furthermore, computational chemistry methods can accurately predict the dipole moment of molecules, further reinforcing the understanding of octane’s nonpolar nature. These computational methods use sophisticated quantum mechanical calculations to model the electronic structure of molecules and predict their properties.
The agreement between experimental measurements and theoretical calculations provides strong evidence for octane’s nonpolar character.
Octane Polarity: Frequently Asked Questions
Octane is a common component of gasoline, and understanding its polarity is crucial in various chemical applications. These FAQs address common questions about the polarity of octane.
What does it mean for a molecule to be polar or nonpolar?
Polarity refers to the uneven distribution of electron density within a molecule. This creates a partial positive and a partial negative charge. If the electron density is evenly distributed, the molecule is nonpolar. The determination if is octane polar lies on its symmetry.
Why is octane considered nonpolar?
Octane is a hydrocarbon comprised of carbon and hydrogen atoms linked by covalent bonds. The electronegativity difference between carbon and hydrogen is small, and the symmetrical structure of octane means any slight dipoles cancel each other out. Thus, is octane polar, NO.
How does octane’s nonpolarity affect its properties?
Because octane is nonpolar, it is not soluble in polar solvents like water. It prefers to dissolve in other nonpolar substances. This also influences its boiling point and other physical properties.
Is the fact that octane is nonpolar important for gasoline?
Yes, the nonpolarity of octane and other hydrocarbons in gasoline is significant. It allows gasoline to mix well with other nonpolar additives and prevents it from separating into layers, leading to more efficient combustion in engines.
Hopefully, this clears up any confusion about whether **is octane polar** or not! Feel free to experiment and explore more about hydrocarbons. Happy chemistry!