The double helix of DNA, a foundational structure in biology, relies heavily on nucleotides. These critical components, deeply studied within the realm of molecular biology, owe their existence to the pioneering work of figures like James Watson. Exploring the multifaceted function of nucleotide reveals its involvement not only in heredity but also in various cellular processes. Understanding the function of nucleotide, therefore, becomes paramount for anyone seeking a comprehensive grasp of life’s underlying mechanisms.
At the heart of every living organism lies a class of organic molecules so fundamental, so versatile, that life as we know it would be impossible without them: nucleotides.
While often immediately associated with their role as the structural units of DNA and RNA – the molecules that carry and translate genetic information – the significance of nucleotides extends far beyond the realm of heredity.
They are not merely the building blocks of our genetic code; they are dynamic players in cellular energy management, intricate signaling cascades, and the catalytic prowess of enzymes.
More Than Just DNA and RNA Components
Nucleotides, in their essence, are composed of three distinct components: a nitrogenous base (either a purine or a pyrimidine), a five-carbon sugar (ribose or deoxyribose), and one or more phosphate groups.
This seemingly simple structure belies an extraordinary functional diversity.
Think of them as molecular chameleons, capable of adapting to a wide array of roles within the cell.
While their contribution to DNA and RNA is undeniably crucial, restricting our understanding of nucleotides to this function alone would be akin to admiring a symphony solely for the individual notes, missing the grand orchestration of life they conduct.
The Nucleotide Thesis: A Symphony of Functions
This article seeks to explore the multifaceted world of nucleotides, revealing their indispensable contribution to various biological processes. We propose the following thesis:
Nucleotides play diverse and critical roles in energy transfer, signaling, and enzymatic reactions, extending their influence far beyond their well-known function as the building blocks of genetic material.
Through a detailed examination of their structure, function, and participation in essential cellular pathways, we aim to illuminate the profound impact of these remarkable molecules on the very fabric of life.
The versatility of nucleotides extends far beyond their individual components. They are the key to life itself, enabling the storage and transmission of genetic information. Let’s delve deeper into their pivotal role in deoxyribonucleic acid, or DNA.
Nucleotides: The Foundation of Genetic Information (DNA Focus)
DNA, often hailed as the blueprint of life, owes its very existence and function to nucleotides. These molecules are not just passive components; they are the architects of heredity, the keepers of our genetic code, and the essential players in the faithful duplication of life’s instructions.
DNA: The Blueprint of Life
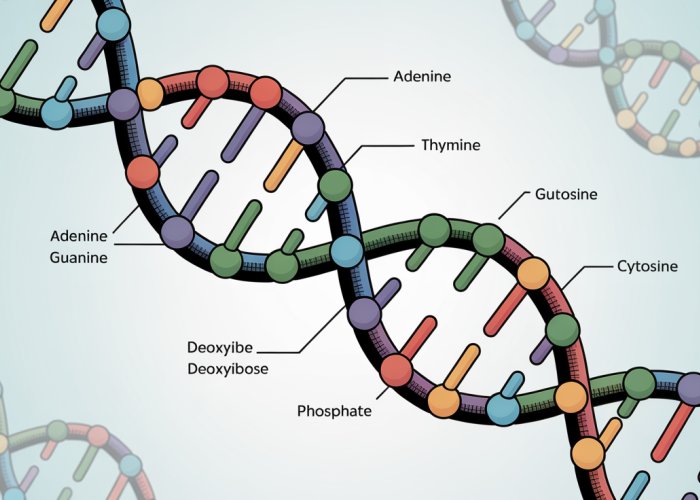
DNA’s structure is an elegant masterpiece of molecular engineering. It is the iconic double helix, a twisted ladder where two strands of nucleotides wind around each other. This unique structure provides both stability and accessibility, crucial for its role in storing and transmitting genetic information.
The Double Helix and Base Pairing
The backbone of each DNA strand is formed by alternating sugar and phosphate groups, creating a strong and resilient framework. Projecting inward from this backbone are the nitrogenous bases: adenine (A), guanine (G), cytosine (C), and thymine (T).
These bases are not arranged randomly; they adhere to a strict pairing rule: adenine always pairs with thymine (A-T), and cytosine always pairs with guanine (C-G). This complementary base pairing is the cornerstone of DNA’s structure and function.
It ensures that the two strands of the double helix are perfectly matched, allowing for accurate replication and transmission of genetic information.
Encoding the Genetic Code
The sequence of nucleotides along a DNA strand encodes the genetic code, the instructions for building and operating a living organism. Each three-nucleotide sequence, called a codon, specifies a particular amino acid, the building block of proteins.
The order of codons dictates the order of amino acids in a protein, ultimately determining its structure and function. This elegant system allows DNA to store an immense amount of information within its relatively simple structure.
The genetic code is universal, meaning that the same codons specify the same amino acids in nearly all organisms, highlighting the common ancestry of all life on Earth.
DNA Replication: Copying the Blueprint
Before a cell can divide, it must accurately duplicate its DNA, ensuring that each daughter cell receives a complete and identical copy of the genetic information. This process, called DNA replication, is critically dependent on nucleotides.
During replication, the double helix unwinds, and each strand serves as a template for the synthesis of a new complementary strand. DNA polymerase, the enzyme responsible for DNA replication, uses free nucleotides to build the new strands, following the base pairing rules (A-T, C-G).
The result is two identical DNA molecules, each consisting of one original strand and one newly synthesized strand.
This semi-conservative replication ensures a high degree of accuracy in the transmission of genetic information from one generation to the next. Without a sufficient supply of nucleotides, DNA replication would grind to a halt, threatening the integrity and survival of the cell.
Encoding the Genetic Code
While DNA holds the primary blueprint, it’s RNA that often steps into the spotlight to carry out its instructions.
Let’s now explore the crucial role of nucleotides in ribonucleic acid (RNA), the versatile molecule responsible for interpreting and executing the genetic code.
Nucleotides: The Foundation of Genetic Information (RNA Focus)
While DNA serves as the long-term storage unit for genetic information, RNA acts as the messenger and the workhorse, translating that information into functional proteins. RNA, like DNA, is composed of nucleotides, but with key structural and functional differences.
RNA Structure and Differences from DNA
RNA, unlike DNA, is typically a single-stranded molecule. This structural difference allows RNA to fold into complex three-dimensional shapes, giving it catalytic and regulatory functions.
The sugar component of RNA nucleotides is ribose, whereas DNA uses deoxyribose (hence the names ribonucleic acid and deoxyribonucleic acid). This seemingly minor difference has significant implications for RNA’s stability and reactivity.
Another key distinction lies in the nitrogenous bases. While RNA, like DNA, contains adenine (A), guanine (G), and cytosine (C), it replaces thymine (T) with uracil (U). Uracil pairs with adenine (A-U) in RNA.
Types of RNA and Their Roles in Protein Synthesis
RNA molecules come in various forms, each with a specific role in the intricate process of protein synthesis. The three primary types are messenger RNA (mRNA), transfer RNA (tRNA), and ribosomal RNA (rRNA).
Messenger RNA (mRNA) carries the genetic information from DNA in the nucleus to the ribosomes in the cytoplasm. It serves as the template for protein synthesis, dictating the sequence of amino acids.
Transfer RNA (tRNA) acts as an adapter molecule, bringing the correct amino acid to the ribosome based on the mRNA sequence. Each tRNA molecule recognizes a specific codon (a three-nucleotide sequence) on the mRNA and carries the corresponding amino acid.
Ribosomal RNA (rRNA) is a major component of ribosomes, the cellular machinery where protein synthesis takes place. rRNA provides the structural framework for the ribosome and also plays a catalytic role in forming peptide bonds between amino acids.
Transcription: From DNA to RNA
The process of transcription is how the information encoded in DNA is copied into RNA. This process relies heavily on nucleotides.
During transcription, an enzyme called RNA polymerase uses a DNA strand as a template to synthesize a complementary RNA strand.
RNA polymerase reads the DNA sequence and adds the corresponding RNA nucleotides (A, U, G, C) to the growing RNA molecule. The resulting RNA transcript carries the genetic information from DNA to the ribosomes, where it can be translated into protein.
Transcription is a fundamental process in gene expression, ensuring that the genetic information stored in DNA is accurately and efficiently converted into functional RNA molecules. Without this process, protein synthesis would not be possible, and life as we know it would not exist.
RNA’s versatility in shuttling genetic information and participating directly in protein synthesis highlights the cell’s intricate choreography. But information alone isn’t enough; cellular processes require energy to occur. This energy, essential for life’s myriad functions, is largely provided by a specific nucleotide.
ATP: The Universal Energy Currency
Adenosine triphosphate, or ATP, stands as the primary energy currency within cells. Its crucial role is facilitating a multitude of biological processes. Think of it as the cell’s readily available power source, fueling everything from muscle contractions to the transmission of nerve impulses.
How ATP Stores and Releases Energy
ATP’s energy storage and release mechanism is elegantly simple, yet profoundly effective.
The molecule comprises adenosine (adenine and ribose) and a triphosphate tail of three phosphate groups.
It is the bonds between these phosphate groups that hold the key to ATP’s energy-storing capabilities.
These bonds are high-energy bonds; when one is broken through hydrolysis (addition of water), energy is released.
Specifically, the terminal phosphate group is cleaved, converting ATP into adenosine diphosphate (ADP) and inorganic phosphate (Pi).
This reaction is exergonic, meaning it releases energy that the cell can then harness to drive various energy-requiring processes.
The released energy isn’t simply heat; it is coupled to specific reactions, making them thermodynamically favorable.
ATP Powering Cellular Processes
The energy released from ATP hydrolysis powers a remarkable range of cellular activities.
Muscle contraction, for example, relies on ATP to facilitate the interaction between actin and myosin filaments, enabling movement.
Nerve impulse transmission depends on ATP to maintain the electrochemical gradients necessary for the propagation of signals along neurons.
Active transport, the movement of molecules against their concentration gradients, is also fueled by ATP.
This is crucial for maintaining cellular homeostasis and transporting essential nutrients.
These are just a few examples.
ATP’s reach extends to nearly every facet of cellular life.
The Central Role of ATP in Metabolism
Metabolism, the sum of all chemical reactions occurring within a cell or organism, is intrinsically linked to ATP.
ATP acts as the central hub in the energy economy of the cell.
Catabolic pathways, which break down complex molecules, often generate ATP.
Conversely, anabolic pathways, which synthesize complex molecules, require ATP as an energy input.
Glycolysis, the breakdown of glucose, and cellular respiration, the process that extracts energy from glucose in the presence of oxygen, both produce ATP.
Photosynthesis in plants also ultimately leads to ATP synthesis.
This ATP then fuels the synthesis of carbohydrates, proteins, lipids, and nucleic acids.
In essence, ATP acts as the bridge between energy-releasing and energy-consuming reactions, maintaining the delicate balance necessary for life. It allows the cell to capture, transfer, and utilize energy efficiently.
GTP: Another Key Player in Energy Transfer and Signaling
While ATP rightly claims the spotlight as the cell’s primary energy currency, guanosine triphosphate, or GTP, quietly plays its own crucial roles in energy transfer and, perhaps even more significantly, in cellular signaling pathways. Understanding GTP’s structure, function, and unique contributions is vital for a complete picture of cellular energetics and communication.
Decoding GTP’s Structure and Function
Like ATP, GTP is a nucleotide composed of a nitrogenous base (guanine), a five-carbon sugar (ribose), and a triphosphate group. The energy, much like ATP, resides within the bonds linking these phosphate groups. Hydrolysis, the breaking of these bonds, releases energy that can be harnessed to drive cellular processes.
However, subtle yet crucial differences distinguish GTP from ATP. The most obvious difference is the nitrogenous base: guanine in GTP versus adenine in ATP. This seemingly small change dictates the specific proteins with which GTP can interact, leading to its specialized roles.
GTP’s Role in Signal Transduction
GTP’s most prominent role lies in signal transduction. G proteins, a family of molecular switches, rely on GTP to relay signals from cell surface receptors to downstream effectors.
The GTPase Cycle: A Molecular Switch
G proteins exist in two states: an active, GTP-bound state and an inactive, GDP-bound state. When a receptor is activated, it promotes the exchange of GDP for GTP on the G protein. This switch to the GTP-bound state activates the G protein, allowing it to interact with and activate other proteins in the signaling pathway.
The G protein’s intrinsic GTPase activity is crucial for regulating the duration of the signal. The G protein will eventually hydrolyze GTP back to GDP, inactivating itself and shutting off the signal. This self-inactivation mechanism is essential for preventing overstimulation and maintaining cellular homeostasis.
This cycle of GTP binding, activation, hydrolysis, and inactivation is the foundation of G protein-mediated signaling, controlling everything from hormone responses to sensory perception.
GTP’s Involvement in Protein Synthesis
Beyond signal transduction, GTP also plays a vital role in protein synthesis, specifically during initiation, elongation, and termination.
For instance, GTP is required for the binding of initiator tRNA to the ribosome, a critical step in starting the translation process. During elongation, GTP is involved in the binding of aminoacyl-tRNAs to the ribosome and in the translocation of the ribosome along the mRNA. GTP hydrolysis provides the energy and directionality needed for these steps to occur accurately and efficiently.
GTP vs. ATP: A Question of Specificity
While both ATP and GTP are nucleotide triphosphates that serve as energy carriers, their roles are not entirely interchangeable. ATP generally fuels a broader range of metabolic processes, while GTP is more specialized for signal transduction and certain aspects of protein synthesis.
This specificity arises from the unique interactions each nucleotide makes with its target proteins. The guanine base of GTP allows it to bind tightly and specifically to G proteins and other GTP-binding proteins, enabling precise control over their activity. This fine-tuned interaction is key to GTP’s role as a signaling molecule.
In essence, ATP is the cell’s general-purpose fuel, while GTP is a more refined and specialized energy source, particularly vital for orchestrating cellular communication and ensuring accurate protein production. Understanding both is crucial to grasping the complexities of cellular life.
G proteins, with their GTP-dependent activation cycles, are just one example of how nucleotides orchestrate cellular communication. But the nucleotide signaling story doesn’t end there. Another key player, derived directly from ATP, enters the scene as a pivotal second messenger.
Cyclic AMP (cAMP): Nucleotides as Second Messengers in Cell Signaling
Cyclic AMP (cAMP) stands as a prime example of how nucleotides extend their influence beyond direct energy transfer to become critical intermediaries in cell signaling pathways. Its discovery revolutionized our understanding of how cells receive and respond to external stimuli. cAMP acts as a crucial second messenger, relaying signals from the cell membrane to the cell’s interior, ultimately influencing a wide range of cellular processes.
The Synthesis of cAMP: A Cyclization Story
cAMP is not directly ingested or transported into the cell; instead, it is synthesized intracellularly in response to extracellular signals. The synthesis of cAMP is catalyzed by the enzyme adenylyl cyclase. This enzyme, typically located on the inner side of the cell membrane, is activated by various signaling molecules, including hormones and neurotransmitters.
Adenylyl cyclase utilizes ATP as a substrate, cleaving two phosphate groups and forming a cyclic phosphodiester bond between the phosphate group and both the 3′ and 5′ carbon atoms of the ribose sugar. This cyclization reaction results in the formation of cAMP, a nucleotide derivative poised to transmit the signal further downstream.
cAMP’s Role in Cell Signaling Pathways
Once synthesized, cAMP diffuses throughout the cell, carrying the signal initiated at the cell surface. It exerts its effects by binding to and activating a variety of intracellular proteins. The most prominent target of cAMP is protein kinase A (PKA), also known as cAMP-dependent protein kinase.
PKA is a tetrameric enzyme consisting of two regulatory subunits and two catalytic subunits. In its inactive state, the regulatory subunits bind to and inhibit the catalytic subunits.
When cAMP binds to the regulatory subunits, it causes a conformational change that releases the catalytic subunits, activating them. These activated catalytic subunits then phosphorylate a diverse array of target proteins, leading to altered cellular function.
cAMP’s Influence on Gene Expression
The impact of cAMP extends to the regulation of gene expression. Activated PKA can translocate into the nucleus and phosphorylate transcription factors, proteins that bind to DNA and control the transcription of specific genes.
One well-characterized example is the cAMP response element-binding protein (CREB). When phosphorylated by PKA, CREB binds to specific DNA sequences called cAMP response elements (CREs) located in the promoter regions of target genes. This binding recruits other transcriptional co-activators, stimulating the transcription of these genes and ultimately leading to increased protein production.
This mechanism allows extracellular signals, mediated by cAMP, to induce long-term changes in cellular behavior by altering the expression of genes involved in a wide range of processes, including cell growth, differentiation, and metabolism.
Signal Transduction and Nucleotides
Having explored the dynamic role of cAMP as a second messenger, it’s clear that nucleotide involvement extends far beyond simple energy transfer. This leads us to examine signal transduction pathways, where nucleotides emerge as critical orchestrators of cellular communication, mediating responses to a vast array of external stimuli.
The Nucleotide-Signal Transduction Nexus
Signal transduction is the process by which a cell converts one kind of signal or stimulus into another. This often involves a cascade of events, with each step carefully regulated to ensure the appropriate cellular response. Nucleotides, in various forms, are central to this intricate system.
Think of signal transduction as a complex relay race. The initial signal, such as a hormone binding to a receptor, is like the starting pistol. But it’s the nucleotides, acting as intermediate runners, that carry the message efficiently and accurately toward the finish line – the final cellular response.
GTPases: Molecular Switches in Signal Relay
One of the most prominent examples of nucleotide involvement in signal transduction is through GTPases, or guanosine triphosphatases. These proteins act as molecular switches, cycling between an active (GTP-bound) and inactive (GDP-bound) state.
The binding and hydrolysis of GTP provides the energy and the on/off mechanism to control the duration and intensity of the signal. This is crucial for regulating processes such as cell growth, differentiation, and movement.
Imagine a light switch: GTP is like flipping the switch on, activating the signaling pathway, while GTP hydrolysis (converting GTP to GDP) is like flipping the switch off, returning the pathway to its resting state.
Receptor Tyrosine Kinases (RTKs) and Nucleotides
Receptor tyrosine kinases (RTKs) are another critical component of signal transduction pathways. Upon activation by a ligand, RTKs initiate a cascade of phosphorylation events.
These phosphorylation events often rely on ATP as the phosphate donor, illustrating another crucial role for nucleotides in these signaling cascades.
ATP-dependent phosphorylation can modify proteins, altering their activity and allowing them to interact with downstream signaling molecules. This is how signals get amplified and diversified.
Nucleotides and the Fine-Tuning of Cellular Responses
The involvement of nucleotides in signal transduction isn’t simply about turning pathways on or off. It’s also about fine-tuning the response, ensuring that the cell reacts appropriately to the strength and duration of the initial signal.
For instance, the levels of cAMP can be tightly regulated by enzymes that synthesize it (adenylyl cyclases) and degrade it (phosphodiesterases). This precise control allows cells to respond rapidly and reversibly to changing environmental conditions.
Furthermore, different cell types can express different combinations of receptors, signaling proteins, and effector enzymes, allowing them to respond in unique ways to the same external signal. This cellular diversity highlights the remarkable adaptability that nucleotide-mediated signaling pathways provide.
Having witnessed the dynamic signaling roles of nucleotides, especially in the context of signal transduction pathways, it becomes evident that their influence extends far beyond just energy transfer and information storage. Indeed, nucleotides are indispensable partners in enzymatic reactions, acting as essential components of coenzymes that dramatically enhance the catalytic prowess of enzymes.
Nucleotides as Coenzymes: Essential Partners for Enzyme Function
Enzymes, the biological catalysts of life, often require assistance to perform their intricate biochemical transformations. This is where coenzymes step in.
Coenzymes are non-protein organic molecules that bind to enzymes and participate directly in the catalytic reaction. While some coenzymes are derived from vitamins, a significant class incorporates nucleotides as essential building blocks.
The Nucleotide-Coenzyme Connection
The marriage of nucleotides and coenzymes is not merely structural; it’s functional. Nucleotides contribute key chemical functionalities that enable coenzymes to:
- Bind substrates more effectively.
- Stabilize reaction intermediates.
- Participate directly in electron transfer or group transfer reactions.
This synergistic relationship between nucleotides and coenzymes unlocks a vast repertoire of biochemical possibilities.
Examples of Nucleotide-Containing Coenzymes
Several crucial metabolic coenzymes rely on nucleotides for their activity:
-
Nicotinamide adenine dinucleotide (NAD+) and Nicotinamide adenine dinucleotide phosphate (NADP+): These coenzymes, built upon an adenine nucleotide, are central to redox reactions. They act as electron carriers in numerous metabolic pathways, including glycolysis, the citric acid cycle, and oxidative phosphorylation.
-
Flavin adenine dinucleotide (FAD): Another adenine nucleotide-based coenzyme, FAD, participates in redox reactions, often accepting electrons in two-electron transfer steps. It plays a vital role in reactions like the oxidation of fatty acids and the removal of double bonds in organic molecules.
-
Coenzyme A (CoA): Featuring an adenosine moiety, CoA is a crucial carrier of acyl groups. It is instrumental in fatty acid metabolism, the citric acid cycle, and the synthesis of various essential molecules. The reactive thiol group (-SH) of CoA forms thioesters with acyl groups, facilitating their transfer between different molecules.
Enhancing Enzymatic Catalysis: A Closer Look
The presence of a nucleotide within a coenzyme structure drastically alters the enzyme’s capabilities.
For example, NAD+ and NADP+‘s nicotinamide ring, derived from the vitamin niacin, is where the redox action occurs. However, the adenine nucleotide portion of the molecule provides a crucial binding site for the enzyme.
This binding ensures proper orientation of the nicotinamide ring within the enzyme’s active site, maximizing the efficiency of electron transfer. Without the adenine nucleotide, the enzyme’s affinity for the coenzyme, and thus its catalytic activity, would be significantly reduced.
Similarly, CoA’s adenosine component facilitates binding to enzymes involved in acyl group transfer. The enzyme recognizes and interacts with the adenosine moiety, positioning the reactive thiol group of CoA precisely to accept or donate acyl groups. This precision is essential for the accurate and efficient synthesis of complex molecules.
Nucleotides: Beyond Structural Components
In essence, nucleotides within coenzymes are not merely structural scaffolds. They contribute actively to the catalytic process by:
- Enhancing substrate binding.
- Stabilizing reaction intermediates.
- Facilitating electron or group transfer.
This multifaceted involvement highlights the critical role of nucleotides in empowering enzymes to orchestrate the biochemical reactions that sustain life.
Having witnessed the dynamic signaling roles of nucleotides, especially in the context of signal transduction pathways, it becomes evident that their influence extends far beyond just energy transfer and information storage. Indeed, nucleotides are indispensable partners in enzymatic reactions, acting as essential components of coenzymes that dramatically enhance the catalytic prowess of enzymes. This deep dive into nucleotide function brings us to the foundational elements upon which these molecules are built: the nitrogenous bases known as purines and pyrimidines.
Purines and Pyrimidines: The Building Blocks Explained
Purines and pyrimidines are the nitrogenous bases that form the very core of nucleotides, the fundamental units of DNA and RNA. These heterocyclic aromatic compounds are the information-carrying components, dictating the genetic code and orchestrating a myriad of biochemical processes. Understanding their structure and properties is essential for comprehending the behavior of nucleotides and nucleic acids.
An Overview of Purines
Purines consist of a two-ring structure: a pyrimidine ring fused to an imidazole ring. This gives them a more complex structure compared to pyrimidines.
The two primary purines found in nucleic acids are:
-
Adenine (A): Plays a crucial role in both DNA and RNA, pairing with thymine (T) in DNA and uracil (U) in RNA. Adenine is also a component of ATP, the cell’s primary energy currency, and several important coenzymes.
-
Guanine (G): Found in both DNA and RNA, guanine pairs with cytosine (C). It is also involved in various signaling pathways and regulatory processes within the cell.
An Overview of Pyrimidines
Pyrimidines, in contrast to purines, feature a single six-membered ring structure. This simpler architecture contributes to their distinct chemical properties and interactions.
The three primary pyrimidines found in nucleic acids are:
-
Cytosine (C): Present in both DNA and RNA, cytosine pairs with guanine (G). It plays a fundamental role in genetic coding.
-
Thymine (T): Primarily found in DNA, thymine pairs with adenine (A). In RNA, thymine is replaced by uracil.
-
Uracil (U): Primarily found in RNA, uracil replaces thymine and pairs with adenine (A).
Key Structural Differences
The most apparent difference between purines and pyrimidines lies in their ring structures. Purines have a fused two-ring system, whereas pyrimidines possess a single ring.
This structural disparity has significant implications for their:
- Size: Purines are larger molecules due to their two-ring structure.
- Hydrogen bonding capacity: The arrangement of nitrogen and oxygen atoms on the rings dictates their ability to form hydrogen bonds, which are critical for base pairing in DNA and RNA.
- Chemical reactivity: The presence of different functional groups and ring systems influences their chemical behavior in various biochemical reactions.
In summary, purines and pyrimidines, with their distinct structures and properties, serve as the foundation for the genetic code. Understanding these fundamental differences is crucial for appreciating the complexity and elegance of molecular biology.
Nucleotide Functions: Frequently Asked Questions
What are the primary functions of nucleotides in the body?
Nucleotides are crucial building blocks. The core function of nucleotide is to form DNA and RNA, the genetic blueprints. Beyond that, they act as energy carriers (like ATP) and signaling molecules.
How does ATP exemplify the energy-carrying function of a nucleotide?
ATP (adenosine triphosphate) is a nucleotide that stores and transports chemical energy within cells. Its structure allows it to release energy when a phosphate group is broken off. This energy then powers various cellular processes. The function of nucleotide ATP is critical for life.
Can you explain the signaling function of a nucleotide in simple terms?
Certain nucleotides act as signaling molecules. They bind to receptors on cells, triggering a cascade of events that regulate cellular activity. The function of nucleotide signaling is important for cellular communication.
Besides DNA, RNA, and energy, what other roles do nucleotides play?
Nucleotides are also involved in enzyme cofactors. These help enzymes carry out their functions. Furthermore, they participate in various metabolic pathways. The function of nucleotide is therefore quite diverse and vital.
So, there you have it – a look at the essential function of nucleotide! Hopefully, this has given you a better understanding. Now go forth and explore the amazing world of biology!