The atomic nucleus, the dense region at the center of an atom, contains neutrons in nucleus and protons. Understanding the role of neutrons in nucleus is crucial for comprehending nuclear stability, investigated by organizations like the International Atomic Energy Agency (IAEA). The number of neutrons in nucleus impacts the isotope of an element, a key concept in nuclear chemistry. Neutron scattering, a powerful experimental technique, provides valuable data on the arrangement and behavior of neutrons in nucleus, offering insights previously not thought possible.
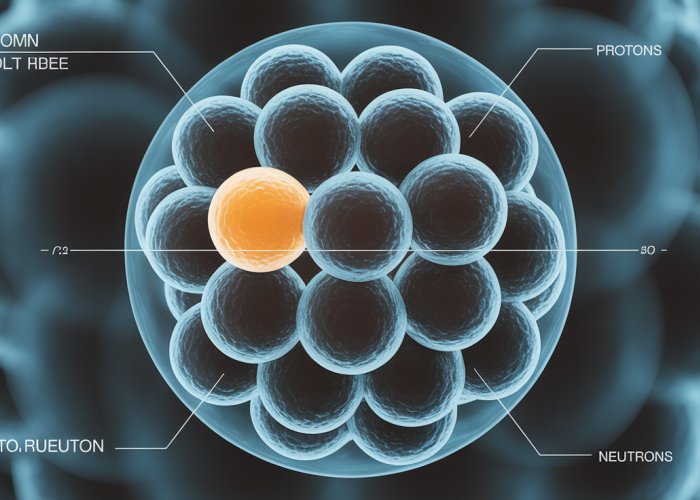
At the heart of every atom, residing in a space of unimaginable density, lies the nucleus. This central core, far from being a simple, uniform mass, is a dynamic realm governed by fundamental forces and composed of the very building blocks of matter.
Within this nuclear domain reside two primary constituents: protons and neutrons.
While electrons orbit the nucleus, it is the protons and neutrons that dictate the atom’s identity and stability. Understanding these nuclear particles is paramount to comprehending the nature of matter itself.
But a crucial question emerges when considering the nucleus:
Why are neutrons so essential for atomic stability?
The Nucleus: A Realm of Concentrated Mass
Imagine the atom as a vast stadium. The nucleus, in this analogy, would be a tiny marble at the center of the field.
Despite its minuscule size, the nucleus houses nearly all of the atom’s mass. This immense concentration of mass gives rise to the powerful forces at play within the nuclear domain.
Protons and Neutrons: The Cornerstones of Matter
Protons, with their positive charge, define the element to which an atom belongs. The number of protons, known as the atomic number, is the unique identifier for each element on the periodic table.
Neutrons, on the other hand, are electrically neutral particles residing alongside protons in the nucleus.
Together, protons and neutrons form the foundation upon which all matter is built.
The Stability Paradox: Why Neutrons Matter
The nucleus presents a puzzle: positively charged protons, packed tightly together, should repel each other with tremendous force.
So, what prevents the nucleus from flying apart?
The answer lies in the crucial role of neutrons. They act as a buffer, mediating the forces between protons and contributing to the strong nuclear force that binds the nucleus together.
Without neutrons, most atomic nuclei would be inherently unstable, and the universe as we know it would not exist. The investigation into this essential particle will reveal the key to understanding the foundation of matter and the forces that govern its existence.
The delicate balance of forces within the nucleus, where protons are crammed together despite their mutual repulsion, begs the question: What holds it all together? The answer lies, in part, with the neutron – a particle whose properties and discovery are central to our understanding of atomic stability.
Decoding the Neutron: Properties and Discovery
The neutron, as its name suggests, is an electrically neutral particle residing within the nucleus of an atom. Unlike its positively charged counterpart, the proton, the neutron carries no electric charge.
Defining the Neutron: Mass, Charge, and Location
Neutrons are found in the nucleus, alongside protons.
They have a mass slightly greater than that of a proton.
This seemingly minor difference in mass plays a crucial role in nuclear stability and radioactive decay processes.
Crucially, neutrons possess no electric charge, differentiating them from positively charged protons and negatively charged electrons.
The lack of charge is critical to their function, as it allows them to penetrate the nucleus without being repelled by the positively charged protons.
James Chadwick: Unveiling the Neutron
The discovery of the neutron is credited to British physicist James Chadwick in 1932.
Chadwick’s experiments involved bombarding beryllium with alpha particles, which resulted in the emission of a previously unknown, highly penetrating radiation.
He meticulously analyzed the properties of this radiation, demonstrating that it consisted of neutral particles with a mass close to that of the proton.
This groundbreaking discovery earned Chadwick the Nobel Prize in Physics in 1935 and revolutionized our understanding of the atom.
Neutrons vs. Protons vs. Electrons: A Comparative Look
To fully grasp the neutron’s significance, it’s important to distinguish it from the other fundamental particles that make up an atom: protons and electrons.
- Protons: Positively charged particles located in the nucleus. The number of protons defines the element.
- Neutrons: Electrically neutral particles also located in the nucleus. Contribute to the mass and stability of the nucleus.
- Electrons: Negatively charged particles orbiting the nucleus. Determine the chemical properties of the atom.
While electrons dictate how atoms interact to form molecules, and protons define the element itself, neutrons play a vital role in stabilizing the nucleus. Without neutrons, most atomic nuclei would be inherently unstable and unable to exist.
Decoding the Neutron: Properties and Discovery set the stage by introducing a key player in the atomic nucleus. However, the neutron doesn’t operate in isolation. Its existence is inextricably linked to that of the proton, forming a nuclear partnership that dictates the identity and stability of elements. Understanding this intricate relationship is crucial to unlocking the secrets of the atom.
Protons and Neutrons: A Nuclear Partnership
The nucleus of an atom is not a chaotic jumble of particles; instead, it is a highly organized structure where protons and neutrons coexist in a delicate balance. Their interplay governs the fundamental properties of the element.
This partnership isn’t merely a matter of co-location. It is an interdependent relationship, where the number of each particle directly impacts the atom’s characteristics and behavior.
The Interdependence of Nuclear Particles
Protons and neutrons don’t exist independently within the nucleus. They are held together by the strong nuclear force, a force that overcomes the electrostatic repulsion between the positively charged protons.
The number of protons dictates the element’s identity. While the number of neutrons contributes to the atom’s mass and stability. Altering either of these quantities results in a completely different atomic configuration.
Atomic Number: The Identity Card of an Element
The atomic number is the defining characteristic of an element. It represents the number of protons found in the nucleus of every atom of that element.
For instance, every atom with six protons is, by definition, a carbon atom. Change the number of protons, and you change the element entirely.
The atomic number is so fundamental that it serves as the element’s unique identifier, its "identity card" within the periodic table. The organization of the periodic table is based on ascending atomic numbers.
Each element is assigned a specific position. This position is determined solely by the number of protons in its nucleus.
Mass Number: Weighing the Nucleus
While the atomic number defines what an element is, the mass number indicates its weight. The mass number is the total number of protons and neutrons in an atom’s nucleus.
Since protons and neutrons contribute nearly all of the atom’s mass, the mass number provides a close approximation of the atom’s atomic mass.
Calculating Mass Number
The calculation is straightforward:
Mass Number = Number of Protons + Number of Neutrons
For example, an atom of carbon-12 (¹²C) has 6 protons and 6 neutrons, giving it a mass number of 12.
Similarly, an atom of uranium-238 (²³⁸U) has 92 protons and 146 neutrons, resulting in a mass number of 238.
Understanding both atomic number and mass number is essential for differentiating between elements and their isotopes, paving the way for a deeper understanding of nuclear chemistry and physics.
Decoding the neutron and understanding its partnership with the proton unlocks a deeper understanding of the elements themselves. But the story doesn’t end there. The number of protons rigidly defines an element, but the number of neutrons? That can vary, leading to a fascinating phenomenon with far-reaching implications.
Isotopes: Exploring Atomic Variations
The atoms of a single element aren’t all created equal. While they share the same defining number of protons, they can differ in their neutron count.
These atomic variations give rise to isotopes, atoms of the same element exhibiting different atomic masses. This seemingly small difference dramatically broadens the range of possibilities within the periodic table and has profound implications across numerous scientific fields.
Defining Isotopes: Same Element, Different Mass
At its core, an isotope is an atom that shares its atomic number (number of protons) with other atoms of the same element. However, it differs in its mass number, which is the sum of protons and neutrons.
This difference stems from a variation in the number of neutrons within the nucleus.
For example, all carbon atoms have six protons, but some have six neutrons (Carbon-12), while others have eight (Carbon-14). These are both isotopes of carbon.
The chemical properties of isotopes are virtually identical, since these are defined by the number and arrangement of electrons.
However, the difference in mass can influence physical properties and, crucially, nuclear stability.
Carbon-12, Carbon-14, and Other Notable Examples
Carbon provides an excellent illustration of isotopes. Carbon-12, with 6 protons and 6 neutrons, is the most abundant and stable isotope of carbon.
Carbon-14, on the other hand, possesses 6 protons and 8 neutrons.
It is a radioactive isotope that decays over time. Hydrogen also has isotopes: Protium (Hydrogen-1), Deuterium (Hydrogen-2), and Tritium (Hydrogen-3).
Uranium is another element with well-known isotopes, Uranium-235 and Uranium-238, which are crucial in nuclear power and weapons.
The Significance of Neutron Count
The number of neutrons in an isotope affects the properties of the atom.
Adding neutrons increases the mass of the atom, but it also can influence the stability of the nucleus.
Some isotopes are stable, meaning they will exist indefinitely. While others are unstable, meaning they will undergo radioactive decay over time.
The stability of an isotope depends on the balance between the strong nuclear force, which holds the nucleus together, and the electrostatic repulsion between protons.
Applications of Isotopes: From Dating the Past to Healing the Present
Isotopes, with their unique properties, have found applications in diverse fields:
- Radiocarbon Dating: Carbon-14’s radioactive decay is the foundation of radiocarbon dating, used to determine the age of organic materials up to approximately 50,000 years old. This technique has revolutionized archaeology and paleontology.
- Medical Imaging: Radioactive isotopes like Technetium-99m are used as tracers in medical imaging techniques such as SPECT scans. These tracers allow doctors to visualize internal organs and detect abnormalities.
- Cancer Treatment: Radioactive isotopes like Iodine-131 are used in radiation therapy to target and destroy cancerous cells.
- Industrial Applications: Isotopes are used in various industrial applications, such as gauging the thickness of materials and tracing the flow of liquids and gases.
The ability to manipulate and detect isotopes has opened up a wealth of possibilities in science, medicine, and industry.
From unveiling the secrets of ancient civilizations to diagnosing and treating diseases, isotopes have proven to be invaluable tools.
Decoding the neutron and understanding its partnership with the proton unlocks a deeper understanding of the elements themselves. But the story doesn’t end there. The number of protons rigidly defines an element, but the number of neutrons? That can vary, leading to a fascinating phenomenon with far-reaching implications.
The Strong Nuclear Force: The Glue That Holds It All Together
The nucleus of an atom, a space packed with positively charged protons, presents a perplexing problem.
According to the fundamental laws of electromagnetism, like charges repel. So, why doesn’t the nucleus simply explode?
The answer lies in the strong nuclear force, a fundamental force of nature that operates at incredibly short distances within the nucleus. It is, in essence, the glue that holds the atomic nucleus together.
Overcoming Electrostatic Repulsion
Protons, crammed together in the tiny confines of the nucleus, experience a powerful electrostatic repulsion. This force, if left unchecked, would cause the nucleus to disintegrate instantly.
The strong nuclear force, however, is significantly stronger than the electrostatic force at these minute distances.
It acts as an attractive force between nucleons (protons and neutrons), effectively counteracting the repulsive forces and maintaining the nucleus’s structural integrity.
The Neutron’s Role in Nuclear Stabilization
While the strong nuclear force acts on both protons and neutrons, neutrons play a particularly crucial role in stabilizing the nucleus.
Neutrons contribute to the strong nuclear force without adding to the electrostatic repulsion.
Think of them as the "shock absorbers" of the nucleus, mediating the interactions between protons and diluting the concentration of positive charge.
The presence of neutrons increases the overall strong nuclear force within the nucleus, making it more stable.
The Neutron-Proton Ratio
The ratio of neutrons to protons is a critical factor in determining the stability of a nucleus.
Lighter elements tend to have a neutron-to-proton ratio close to 1:1.
However, as the atomic number increases, a higher proportion of neutrons is required to maintain stability.
This is because the electrostatic repulsion between protons becomes increasingly significant in larger nuclei, demanding a greater contribution from the strong nuclear force.
A Delicate Balance of Forces
The stability of an atomic nucleus hinges on a delicate balance between the strong nuclear force and the electrostatic force.
If the repulsive forces outweigh the attractive forces, the nucleus becomes unstable and may undergo radioactive decay.
This decay process involves the emission of particles or energy, ultimately transforming the unstable nucleus into a more stable configuration.
Understanding the strong nuclear force and the role of neutrons is, therefore, paramount to comprehending the behavior and stability of matter itself.
The nucleus is a delicate dance of opposing forces. The strong nuclear force strives to bind protons and neutrons together, while the electrostatic force between protons pushes them apart. This intricate interplay determines not only the stability of an atom, but also its very identity. An imbalance in this nuclear equation can lead to fascinating, and sometimes unstable, consequences.
Atomic Mass, Radioactivity, and Nuclear Instability
Defining the Atomic Mass Unit (AMU)
When dealing with the incredibly small masses of atoms and their constituent particles, using grams or kilograms becomes impractical. Enter the atomic mass unit (AMU), a standardized unit designed for measuring the mass of atomic and subatomic particles.
One AMU is defined as 1/12th of the mass of a neutral carbon-12 atom. This provides a convenient scale where the mass of a proton and a neutron are each approximately 1 AMU.
The AMU allows scientists to express atomic masses in a manageable way. It’s essential for understanding the subtle mass differences between isotopes and for performing calculations related to nuclear reactions.
Radioactivity: When the Nucleus Falls Apart
Radioactivity is a phenomenon where an unstable atomic nucleus spontaneously decays, emitting particles and energy in the process. This decay occurs because the nucleus is trying to reach a more stable configuration.
The underlying cause of radioactivity often boils down to an imbalance in the neutron-to-proton ratio. Certain combinations of protons and neutrons are inherently more stable than others.
If a nucleus has too many or too few neutrons relative to its number of protons, the strong nuclear force may not be sufficient to overcome the electrostatic repulsion. This leads to instability and, ultimately, radioactive decay.
Types of Radioactive Decay
There are several types of radioactive decay, each characterized by the specific particles emitted and the changes that occur within the nucleus.
-
Alpha decay involves the emission of an alpha particle (two protons and two neutrons, equivalent to a helium nucleus). This reduces both the atomic number (number of protons) and the mass number (total number of protons and neutrons) of the atom.
-
Beta decay involves the conversion of a neutron into a proton (or vice versa), along with the emission of a beta particle (an electron or a positron) and a neutrino (or antineutrino). This changes the atomic number but not the mass number.
-
Gamma decay involves the emission of high-energy photons (gamma rays) from an excited nucleus. This does not change the atomic number or the mass number, but it releases excess energy from the nucleus.
The Neutron Connection to Radioactivity
The number of neutrons in an isotope directly influences its stability and, consequently, its likelihood of undergoing radioactive decay. Isotopes with neutron-to-proton ratios that deviate significantly from the "band of stability" are more likely to be radioactive.
For example, Carbon-14, with 6 protons and 8 neutrons, is an isotope of carbon that undergoes beta decay, transforming into Nitrogen-14. This is because the neutron-to-proton ratio in Carbon-14 is higher than what is energetically favorable, driving the decay process.
The closer an isotope’s neutron-to-proton ratio is to the optimal range, the more stable it tends to be. Conversely, isotopes with extreme neutron excesses or deficiencies are almost always radioactive.
This principle underlies many applications of radioactive isotopes, such as radiocarbon dating, where the decay rate of Carbon-14 is used to determine the age of ancient organic materials. It also helps to understand nuclear processes occurring in nuclear reactors.
Ernest Rutherford: Pioneer of Nuclear Physics
The journey into the heart of the atom would be incomplete without acknowledging the monumental contributions of Ernest Rutherford. He stands as a towering figure in the history of science, a true pioneer whose groundbreaking experiments fundamentally reshaped our understanding of matter.
His meticulous work not only led to the discovery of the nucleus, but also laid the groundwork for the field of nuclear physics as we know it today.
Rutherford’s Revolutionary Experiments
Rutherford’s most famous experiment, the gold foil experiment, conducted in 1909, provided the first direct evidence of the nucleus. This pivotal experiment involved firing alpha particles (helium nuclei) at a thin gold foil.
According to the prevailing "plum pudding model" of the atom at the time, these particles should have passed straight through with minimal deflection.
Instead, Rutherford and his team observed that a small fraction of the alpha particles were deflected at large angles, some even bouncing directly back.
This unexpected result led Rutherford to conclude that the atom’s positive charge and most of its mass were concentrated in a tiny, dense core – the nucleus.
The Birth of Nuclear Physics
This discovery was revolutionary. It overturned the existing model of the atom and provided a new framework for understanding atomic structure.
Rutherford’s model, with its central nucleus surrounded by orbiting electrons, became the cornerstone of modern atomic theory.
Beyond the discovery of the nucleus, Rutherford also made significant contributions to the understanding of radioactive decay. He identified and named alpha and beta particles, demonstrating that radioactivity involved the transmutation of one element into another.
This realization had profound implications, suggesting that the atom was not indivisible, but could be altered.
Legacy and Impact
Rutherford’s impact on nuclear physics is immeasurable. His work paved the way for countless discoveries. These range from the identification of the proton to the development of nuclear energy and medical isotopes.
He also mentored a generation of brilliant physicists, including James Chadwick (discoverer of the neutron) and Niels Bohr (who refined the model of the atom).
Ernest Rutherford’s legacy endures, not only through his scientific achievements, but also through the inspiration he provided to future generations of scientists. He left an indelible mark on the landscape of physics. His relentless pursuit of knowledge and his ability to challenge existing paradigms continue to inspire scientists today.
Ernest Rutherford’s insights paved the way for a deeper understanding of the atom, but the story doesn’t end there. Unlocking the secrets of the nucleus required a more complete picture, one where the role of neutrons comes into sharp focus. We now turn our attention to the significance of these neutral particles and why their presence is far more crucial than one might initially suspect.
Why Neutrons Matter: Implications and Applications
Neutrons, often overlooked compared to their positively charged counterparts, are far from passive bystanders within the nucleus. They are, in fact, essential for the very existence and stability of matter as we know it. Understanding their role unlocks a deeper comprehension of nuclear physics and opens doors to a wide array of practical applications.
The Indispensable Role of Neutrons in Nuclear Stability
The nucleus is a crowded space, packed with positively charged protons that naturally repel each other due to electrostatic forces. Without a counteracting force, the nucleus would simply fly apart. This is where neutrons step in, acting as the glue that holds the nucleus together.
They contribute to the strong nuclear force, a powerful attractive force that overcomes the electrostatic repulsion between protons. The presence of neutrons effectively dilutes the concentration of positive charges, increasing the distance between protons and thus reducing the repulsive force between them.
Neutrons act as a buffer between protons, minimizing proton-proton interactions, and increasing the nuclear force overall. Without sufficient neutrons, the repulsive forces overwhelm the attractive forces, leading to nuclear instability and ultimately, radioactive decay.
Applications Across Disciplines
The importance of neutrons extends far beyond the realm of theoretical physics. A thorough understanding of their properties and behavior has led to groundbreaking advancements in various scientific and technological fields.
Nuclear Energy
Nuclear power plants harness the energy released during nuclear fission, a process in which heavy nuclei are split into smaller ones. Neutrons play a critical role in initiating and sustaining these chain reactions. Controlled neutron bombardment of fissile materials, such as uranium, leads to the release of energy, which is then used to generate electricity.
Medical Isotopes
Many medical isotopes, used for both diagnostic imaging and therapeutic treatments, are produced through neutron bombardment of stable isotopes. For example, Technetium-99m, a widely used medical isotope for imaging, is produced by bombarding molybdenum with neutrons in a nuclear reactor. These isotopes are invaluable tools for detecting diseases, monitoring treatment progress, and even targeting cancerous cells.
Materials Science
Neutron scattering techniques are employed to study the structure and properties of materials at the atomic level. Unlike X-rays, which interact primarily with electrons, neutrons interact with atomic nuclei, providing complementary information about the arrangement and dynamics of atoms within a material. This technique is crucial for developing advanced materials with tailored properties for various applications.
Radiocarbon Dating
Carbon-14, a radioactive isotope of carbon, is used in radiocarbon dating to determine the age of organic materials. Carbon-14 is produced in the atmosphere through interactions between neutrons and nitrogen atoms. The ratio of Carbon-14 to Carbon-12 in a sample decreases over time as Carbon-14 undergoes radioactive decay. By measuring this ratio, scientists can accurately determine the age of ancient artifacts, fossils, and geological samples.
The Ongoing Quest for Knowledge
While we have made significant progress in understanding the role of neutrons, many questions remain unanswered. Further research is needed to fully unravel the complexities of nuclear structure and the fundamental forces that govern it. Continuing to explore the neutron’s role will undoubtedly lead to new discoveries and innovations in the years to come.
Frequently Asked Questions: Neutrons in the Nucleus
Here are some common questions about neutrons and their role within the nucleus of an atom.
What exactly is a neutron?
A neutron is a subatomic particle found in the nucleus of an atom. It has no electrical charge (it’s neutral) and has roughly the same mass as a proton.
Why are neutrons important in the nucleus?
Neutrons are essential for the stability of the nucleus. They contribute to the strong nuclear force, which counteracts the repulsive force between protons, holding the nucleus together. The presence of neutrons in nucleus is crucial for heavier elements to exist.
How do neutrons affect the atomic mass of an element?
The atomic mass of an element is determined by the total number of protons and neutrons in its nucleus. Since neutrons have mass, they contribute significantly to the atomic mass.
Can the number of neutrons change in an atom?
Yes, atoms of the same element can have different numbers of neutrons. These variations are called isotopes. Isotopes have the same number of protons but different numbers of neutrons in the nucleus, leading to different atomic masses.
So, there you have it – your beginner’s guide to understanding neutrons in nucleus! Hopefully, this gives you a solid foundation. Now go forth and impress your friends with your newfound knowledge! Always remember your neutrons in nucleus.