The understanding of nacl intermolecular forces is fundamental to predicting material properties. Ionic bonding, a primary attribute of NaCl, dictates the strong electrostatic interactions at play. These forces are significantly impacted by the Debye length, a measure of charge screening in ionic solutions. Researchers at the National Institute of Standards and Technology (NIST) frequently employ techniques like Molecular Dynamics simulations to further characterize these interactions, highlighting their importance in various scientific domains. This guide offers a comprehensive exploration of nacl intermolecular forces, delving into the factors that influence their strength and behavior.
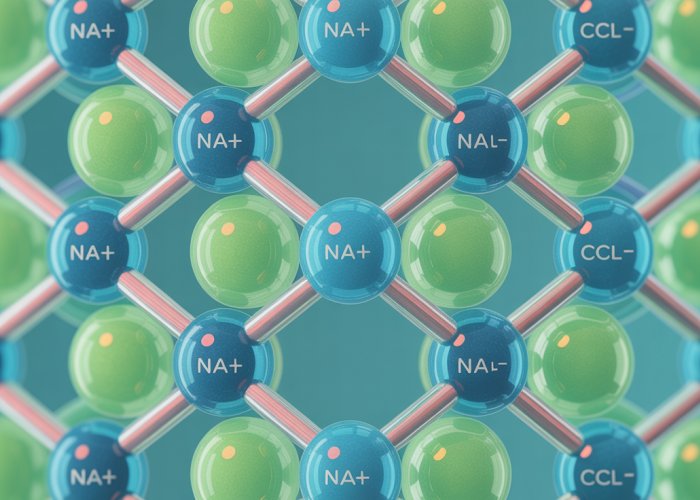
Sodium chloride (NaCl), more commonly known as table salt, is a cornerstone of both our daily lives and numerous scientific disciplines.
Its prevalence extends far beyond the kitchen, playing a crucial role in biological processes, industrial applications, and chemical research.
Understanding the forces that govern its structure and behavior is, therefore, of paramount importance.
This article aims to provide a comprehensive exploration of the intermolecular forces at play within sodium chloride.
While often discussed in the context of ionic bonding, a deeper dive reveals a complex interplay of electrostatic interactions that dictate its properties.
The Ubiquitous Nature of Sodium Chloride
From seasoning our food to maintaining electrolyte balance in our bodies, sodium chloride is truly ubiquitous.
Its simple chemical formula belies the intricate network of forces that hold it together.
This makes it an ideal model for understanding fundamental principles of chemical bonding.
Purpose and Scope
The central goal of this discussion is to elucidate the intermolecular forces that govern the behavior of NaCl.
We will explore how these forces influence its crystal structure, its dissolution in water, and its interactions with other substances.
While ionic bonding takes center stage, we will also touch on the role of weaker intermolecular interactions.
Why Understanding NaCl Forces Matters
The significance of understanding the forces within sodium chloride extends across a wide range of scientific fields.
In chemistry, it provides a foundational understanding of ionic bonding and crystal structures.
In biology, it is essential for comprehending electrolyte balance, nerve impulse transmission, and cellular function.
In materials science, the properties of NaCl serve as a basis for designing and understanding other ionic compounds and crystalline materials.
Ultimately, grasping the nuances of these forces unlocks deeper insights into the behavior of matter itself.
Sodium chloride’s journey from a simple crystal to a dissolved solution hinges on the intricate forces at play between its constituent ions. But to truly grasp the nature of these interactions, it’s essential to first differentiate between the types of forces we’ll be exploring, clarifying our focus on intermolecular forces rather than intramolecular ones.
Ionic vs. Intermolecular Forces: Setting the Stage
Within the realm of chemical interactions, distinguishing between intermolecular and intramolecular forces is paramount. While both contribute to the overall behavior of a substance, they operate at different levels and with varying strengths.
Intramolecular Forces: Holding the Molecule Together
Intramolecular forces are those that hold atoms together within a molecule. In the case of sodium chloride, this refers primarily to the ionic bond between the sodium (Na+) and chloride (Cl-) ions. This bond arises from the electrostatic attraction between oppositely charged ions, resulting in the formation of a stable molecule.
It’s important to acknowledge that our discussion will not delve deeply into the quantum mechanical intricacies of ionic bond formation itself, such as electron sharing or transfer. Instead, we will accept its presence as a given and explore the consequences of this strong internal bond.
Intermolecular Forces: Interactions Between Molecules
In contrast, intermolecular forces are the attractions and repulsions that occur between molecules. While sodium chloride exists as a lattice of ions rather than discrete molecules in its solid state, the term "intermolecular" is still broadly applicable to describe the interactions between these ions and other species, like water molecules.
These forces are generally weaker than intramolecular forces, but they are still crucial for determining physical properties such as melting point, boiling point, and solubility.
The Dominance of Ionic Bonds
It’s undeniable that ionic bonds reign supreme in dictating NaCl’s fundamental properties. The intense electrostatic attraction between Na+ and Cl- ions is what gives sodium chloride its high melting point, its crystalline structure, and its overall stability in solid form.
However, the story doesn’t end there. While ionic bonds are the primary force holding the crystal together, intermolecular forces dictate how it interacts with its environment.
The Role of Electrostatic Forces
Electrostatic forces are at the heart of all interactions involving sodium chloride. They are responsible for the attraction between the positively charged sodium ions and the negatively charged chloride ions, as well as the interactions between these ions and polar molecules like water.
The magnitude of these forces is governed by Coulomb’s Law, which we will delve into later. Understanding electrostatic forces is therefore crucial to understanding everything from the stability of the crystal lattice to the dissolution of sodium chloride in water.
Electrostatic Attraction: The Foundation of NaCl’s Structure
Having established the distinction between the internal forces holding individual ions together and the interactions between them, we now turn our attention to the primary force responsible for NaCl’s very existence as a stable, solid compound: electrostatic attraction. This fundamental force governs the interaction between the positively charged sodium ions (Na+) and the negatively charged chloride ions (Cl-), dictating the arrangement and stability of the entire crystal lattice.
The Nature of Electrostatic Forces in NaCl
The electrostatic force between ions arises from their opposing electrical charges. Sodium, having lost an electron, carries a positive charge (+1e), while chlorine, having gained an electron, carries a negative charge (-1e).
This difference in charge creates an electrical field, and the interaction between these fields is what we perceive as electrostatic attraction. These forces are not merely present; they are the dominant factor determining the structure and properties of sodium chloride.
Coulomb’s Law: Quantifying the Attraction
The magnitude of this electrostatic force can be precisely calculated using Coulomb’s Law, a cornerstone of electrostatics. Coulomb’s Law states that the force (F) between two point charges is directly proportional to the product of the magnitudes of the charges (q1 and q2) and inversely proportional to the square of the distance (r) between them.
The formula for Coulomb’s Law is:
F = k (q1 q2) / r²
Where ‘k’ is Coulomb’s constant. This constant reflects the strength of the electrostatic force in a vacuum.
Decoding the Formula: Variables Explained
Let’s break down each component of Coulomb’s Law:
-
F: Represents the electrostatic force between the ions, measured in Newtons (N). A positive value indicates a repulsive force, while a negative value indicates an attractive force. In the case of NaCl, the force is attractive.
-
k: Coulomb’s constant (approximately 8.9875 × 10^9 N⋅m²/C²). This constant accounts for the permittivity of free space and ensures the equation’s dimensional consistency.
-
q1 and q2: These denote the magnitudes of the charges on the two ions, measured in Coulombs (C). For Na+ and Cl-, these charges are equal in magnitude but opposite in sign.
-
r: Represents the distance between the centers of the two ions, measured in meters (m). This is the most crucial variable, as the force is inversely proportional to its square.
The Inverse Square Law and Its Implications
The inverse square relationship between force and distance is a key takeaway. As the distance between the sodium and chloride ions increases, the electrostatic force between them decreases dramatically. Conversely, as the ions get closer, the force intensifies rapidly.
This principle dictates the optimal spacing between ions in the NaCl crystal lattice.
Coulomb’s Law and NaCl Stability
The stability of the NaCl crystal lattice is a direct consequence of the balance between attractive and repulsive forces governed by Coulomb’s Law. The arrangement of ions in the lattice maximizes attractive forces between oppositely charged ions while minimizing repulsive forces between ions of the same charge.
Any deviation from this optimal arrangement would result in a decrease in the overall electrostatic attraction, leading to a less stable, higher-energy configuration.
This inherent drive toward minimal energy ensures that sodium chloride adopts its characteristic crystalline structure. The strength of the electrostatic attraction, as defined by Coulomb’s Law, is therefore not just a theoretical calculation but the very foundation upon which the stability of NaCl rests.
Having quantified the electrostatic attraction between individual sodium and chloride ions through Coulomb’s Law, the question naturally arises: how do countless such interactions manifest on a macroscopic scale to form the seemingly simple yet remarkably stable structure of table salt? The answer lies in the elegant and efficient arrangement of ions within the crystal lattice.
The Crystal Lattice: An Ordered Arrangement
The most visually striking characteristic of sodium chloride, beyond its simple chemical formula, is its crystalline structure. It’s not merely a random aggregation of ions; it’s a highly ordered, three-dimensional array known as a crystal lattice.
This specific arrangement directly results from the interplay of attractive and repulsive electrostatic forces between the ions.
The Face-Centered Cubic Structure
Sodium chloride adopts a face-centered cubic (FCC) lattice structure. In this arrangement, each sodium ion (Na+) is surrounded by six chloride ions (Cl-), and conversely, each chloride ion is surrounded by six sodium ions.
This creates a repeating pattern that extends throughout the entire crystal. Imagine a cube where sodium and chloride ions alternate positions at each corner and at the center of each face.
The visual representation of this is a three-dimensional grid, with each ion held firmly in place by the powerful electrostatic forces.
Minimization of Potential Energy: The Driving Force
The formation of the crystal lattice isn’t accidental; it’s driven by a fundamental principle of physics: the minimization of potential energy. Ions arrange themselves in a way that minimizes their overall potential energy, leading to a state of maximum stability.
In the NaCl lattice, the arrangement ensures that each ion is surrounded by the maximum number of oppositely charged ions while minimizing contact with similarly charged ions.
This balance maximizes the attractive forces and minimizes the repulsive forces, thereby lowering the overall energy of the system.
Any deviation from this ordered arrangement would increase the potential energy, making the structure less stable and less likely to exist.
Energetic Stability: A Measure of Strength
The crystal lattice structure imparts significant energetic stability to sodium chloride. The strong electrostatic forces between the ions require a considerable amount of energy to overcome.
This is reflected in NaCl’s high melting point (801°C) and boiling point (1,413°C). It takes significant thermal energy to disrupt the highly ordered arrangement and overcome the powerful ionic bonds.
The lattice energy is a measure of this stability – it represents the energy released when gaseous ions combine to form the solid crystal lattice.
A large negative lattice energy indicates a highly stable crystal structure.
The Symphony of Ionic Bonds and Electrostatic Forces
The crystal lattice is a direct consequence of the powerful ionic bonds and the ubiquitous electrostatic forces between the sodium and chloride ions. Ionic bonds are essentially the result of the electrostatic attraction.
The lattice formation is not merely a static arrangement. It represents a dynamic equilibrium where the ions are constantly vibrating around their fixed positions.
However, the electrostatic forces are strong enough to maintain the overall structural integrity of the lattice, even at relatively high temperatures.
The precise balance between attraction and repulsion, dictated by Coulomb’s Law, determines the specific geometry of the crystal lattice and contributes to the macroscopic properties that we observe in sodium chloride, like its hardness and brittleness.
Having established the robust nature of the sodium chloride crystal lattice, bound by strong electrostatic forces, it’s equally important to consider what happens when this seemingly unbreakable structure encounters a different environment: water. The dissolution of NaCl in water is a prime example of intermolecular forces in action, showcasing a dynamic interplay between ionic bonds and the unique properties of the solvent.
Dissolution and Hydration: NaCl in Solution
The Dissolution Process: From Crystal to Aqueous Ions
When sodium chloride is introduced to water, a fascinating process unfolds. The solid crystal structure begins to break down, and the individual sodium (Na+) and chloride (Cl-) ions become dispersed throughout the water.
This process, known as dissolution, is not merely a physical separation; it’s a chemical interaction driven by the inherent properties of both NaCl and H2O.
Water molecules bombard the crystal surface, and begin to pry away individual ions from the lattice.
Hydration and Solvation: Water’s Embrace
As ions detach from the crystal lattice, they don’t simply float freely in the water. Instead, they undergo hydration, a specific type of solvation where water molecules surround and interact with the ions.
The Orientation of Water Molecules
Water molecules are polar, possessing a slightly negative charge (δ-) on the oxygen atom and slightly positive charges (δ+) on the hydrogen atoms.
This polarity is crucial for hydration. The negatively charged chloride ions (Cl-) attract the partially positive hydrogen atoms of water, while the positively charged sodium ions (Na+) attract the partially negative oxygen atoms.
Hydration Shells: A Sphere of Water
As a result, each ion becomes surrounded by a sphere of water molecules, known as a hydration shell. This shell effectively shields the ion from interacting directly with other ions of opposite charge, preventing them from immediately recombining and reforming the crystal lattice.
The number of water molecules in a hydration shell, as well as how tightly bound they are, depend on several factors including the ion’s charge density and size.
Water’s Polarity: The Key to Dissolution
The polarity of water is the driving force behind NaCl’s dissolution. Without this property, water molecules would not be able to effectively interact with the ions and overcome the strong electrostatic forces holding the crystal lattice together.
Water’s ability to act as a solvent for ionic compounds like NaCl highlights the importance of molecular structure and charge distribution in determining a substance’s properties.
The Impact of Water’s Dielectric Constant
Water possesses a high dielectric constant, a measure of its ability to reduce the electrostatic field between charged particles. This property is critical in weakening the ionic attractions within the NaCl crystal.
Reducing Electrostatic Interactions
A high dielectric constant means that water can effectively diminish the force of attraction between the Na+ and Cl- ions. This reduction in force makes it easier for water molecules to separate the ions from the crystal lattice.
In essence, water acts as a buffer, weakening the electrostatic "glue" that holds the crystal together, thereby facilitating dissolution.
Even within a structure as dominated by ionic bonding as sodium chloride, it’s an oversimplification to suggest that only these forces are present. While the ionic bonds between sodium and chloride ions are overwhelmingly the most significant, weaker intermolecular forces do exist and contribute, albeit minimally, to the overall properties of the compound.
Beyond Ionic Bonds: Acknowledging Weaker Intermolecular Interactions
While ionic bonds dictate the fundamental structure and behavior of NaCl, exploring the realm of weaker intermolecular interactions provides a more nuanced understanding.
These forces, though significantly less potent than ionic attractions, are ubiquitous in chemical systems and can subtly influence properties like crystal packing, surface interactions, and behavior in non-aqueous environments.
The Landscape of Intermolecular Forces
Intermolecular forces are attractive or repulsive forces between molecules.
These forces are generally much weaker than intramolecular forces (ionic, covalent, and metallic bonding).
They are responsible for many physical properties of matter like boiling and melting points, viscosity, and surface tension.
Several types of intermolecular forces exist:
-
Van der Waals Forces: A general term encompassing dipole-dipole, dipole-induced dipole, and London dispersion forces.
-
Hydrogen Bonding: A particularly strong type of dipole-dipole interaction. Although vital in systems containing O-H, N-H, or F-H bonds, it is generally not significant in pure NaCl.
Van der Waals Forces in NaCl: A Minor Role
Van der Waals forces arise from temporary fluctuations in electron distribution, creating transient dipoles.
These forces are distance-dependent and become negligible at larger separations.
In NaCl, the complete charges on the ions and the stable crystal lattice structure mean that the relatively weak, temporary dipoles that cause Van der Waals forces contribute very little to the overall stability or properties of the solid.
Their effect is far overshadowed by the powerful electrostatic interactions.
London Dispersion Forces: An Inherent Presence
Even in nonpolar molecules, temporary fluctuations in electron density can create instantaneous dipoles, leading to attractive forces.
These are termed London dispersion forces.
While NaCl is ionic, the electron clouds surrounding the ions can still experience these fluctuations.
However, their contribution to the overall attractive forces is minimal compared to the electrostatic attraction between the fully charged ions.
Implications of Weak Interactions
Although the influence of these weaker forces is small, they aren’t entirely inconsequential.
They can subtly affect the surface properties of NaCl crystals, potentially influencing how they interact with other materials or adsorb molecules from the air.
They also play a more pronounced role in non-ideal conditions, such as high temperatures or when NaCl is dissolved in specific non-aqueous solvents, situations where the dominant ionic interactions may be somewhat weakened or disrupted.
Even within a structure as dominated by ionic bonding as sodium chloride, it’s an oversimplification to suggest that only these forces are present. While the ionic bonds between sodium and chloride ions are overwhelmingly the most significant, weaker intermolecular forces do exist and contribute, albeit minimally, to the overall properties of the compound.
The relative strength of these interactions, whether ionic or intermolecular, is not fixed but rather depends on a variety of factors. Understanding these factors provides a more complete picture of the behavior of NaCl in different environments and conditions.
Factors Affecting the Strength of Forces in NaCl
The forces that govern the structure and behavior of sodium chloride are not static; their strength is subject to a variety of influences.
These influences range from the intrinsic properties of the ions themselves, such as their charge and size, to external factors such as the surrounding medium.
A careful consideration of these elements is crucial for understanding NaCl’s properties in different contexts.
Distance Between Ions: A Key Determinant
The distance between sodium and chloride ions is a primary determinant of the strength of the electrostatic attraction between them.
This relationship is directly expressed in Coulomb’s Law, where the electrostatic force is inversely proportional to the square of the distance separating the charges.
In simpler terms, as the distance between the ions increases, the attractive force diminishes rapidly.
Within the crystal lattice, the ions are arranged to minimize this distance, thereby maximizing the overall stability of the structure.
Perturbations to this arrangement, such as those caused by thermal energy or external forces, can weaken the ionic bonds.
The Impact of Charge Magnitude
The magnitude of the charges on the sodium and chloride ions (+1 and -1, respectively) plays a crucial role in the strength of the ionic bonds.
According to Coulomb’s Law, the electrostatic force is directly proportional to the product of the charges.
Therefore, ions with higher charges would exhibit significantly stronger electrostatic attractions.
In the case of NaCl, the +1 and -1 charges establish a strong, but not exceptionally high, electrostatic attraction.
If we were to consider a hypothetical compound with doubly or triply charged ions, the lattice energy, and therefore the overall strength of the interactions, would increase dramatically.
The Role of the Surrounding Medium: Solvation Effects
The surrounding medium has a significant impact on the strength of the forces within NaCl, particularly when dissolved in water.
Water, being a polar solvent, effectively weakens the ionic bonds through a process called solvation.
Water’s Dielectric Constant
Water’s high dielectric constant reduces the electrostatic forces between the sodium and chloride ions, facilitating their separation and dispersion throughout the solution.
The water molecules surround the ions, with the oxygen atoms (partially negative) oriented towards the sodium ions and the hydrogen atoms (partially positive) oriented towards the chloride ions.
This hydration process effectively shields the ions from each other, further reducing the strength of their attraction.
Implications for Dissolution
The combined effect of water’s polarity and its high dielectric constant makes it an excellent solvent for ionic compounds like NaCl.
Without these properties, the strong ionic bonds would prevent the compound from dissolving effectively.
Real-World Applications and Significance of NaCl Forces
Having explored the intricate forces at play within sodium chloride, from the dominant ionic bonds to the subtler intermolecular interactions and the factors that influence their strength, it’s time to turn our attention to the tangible impact these forces have on our world. Understanding these interactions is not merely an academic exercise; it’s fundamental to advancements across diverse scientific disciplines and has profound practical implications.
The Ubiquity of NaCl Forces: Interdisciplinary Importance
The importance of understanding the forces within NaCl spans chemistry, biology, and materials science. This understanding enables researchers and professionals to address complex challenges and innovate across various industries.
In chemistry, a grasp of these forces is critical for predicting and controlling chemical reactions involving salts. It provides insight into solubility, reaction rates, and the formation of new compounds.
In biology, the role of NaCl and its constituent ions is paramount in maintaining cellular function, nerve impulse transmission, and fluid balance. Understanding the interactions between ions and biological molecules is crucial for studying these processes.
In materials science, the properties of materials containing NaCl, such as cement and certain ceramics, are directly influenced by the interactions between the ions. This knowledge is vital for developing new materials with tailored properties.
Biological Processes: The Salty Secrets of Life
The forces governing NaCl behavior are deeply entwined with fundamental biological processes. Sodium and chloride ions are key players in maintaining osmotic balance.
They are also critical for nerve impulse transmission and muscle contraction.
Osmotic Balance
Cells maintain a delicate balance of water and electrolytes. NaCl plays a critical role in regulating osmotic pressure across cell membranes.
This ensures cells neither swell nor shrink due to water influx or efflux. Disruptions in this balance can have severe consequences for cellular function and organismal health.
Nerve Impulse Transmission
The transmission of nerve impulses relies on the controlled movement of sodium and potassium ions across neuron membranes.
This generates electrical signals that propagate along nerve fibers. Understanding the forces driving ion transport is essential for unraveling the complexities of the nervous system.
Muscle Contraction
Muscle contraction is another vital process dependent on ion gradients. The influx of sodium ions into muscle cells triggers a cascade of events leading to the interaction of actin and myosin filaments.
This interaction results in the shortening of muscle fibers and the generation of force. The precise control of ion concentrations is crucial for proper muscle function.
Industrial Applications: From Production to Preservation
Beyond biological systems, the understanding of NaCl forces plays a key role in various industrial applications.
Chemical Manufacturing
NaCl serves as a crucial feedstock in the production of numerous chemicals, including chlorine gas, sodium hydroxide (caustic soda), and hydrochloric acid.
Electrolysis, a process driven by understanding ionic forces, breaks down NaCl into its constituent elements, forming the basis for many industrial processes.
Food Preservation
The ability of NaCl to inhibit microbial growth has made it a staple in food preservation for centuries. By drawing water out of bacterial cells through osmosis, NaCl creates an environment hostile to microbial proliferation.
This prolongs the shelf life of various food products.
Materials Engineering
The properties of cement, a widely used construction material, are influenced by the presence of NaCl. Small amounts of NaCl can affect the setting time and strength development of cement.
Understanding these interactions allows engineers to tailor cement formulations for specific applications.
De-icing
The ability of NaCl to lower the freezing point of water makes it an effective de-icing agent for roads and walkways.
By disrupting the formation of ice crystals, NaCl helps to prevent the buildup of hazardous ice layers. This ensures safer transportation during winter months.
FAQs: Understanding NaCl Intermolecular Forces
Here are some frequently asked questions regarding the intermolecular forces present in sodium chloride (NaCl) and their implications.
What type of intermolecular forces exist in NaCl?
The primary force in NaCl is not intermolecular, but ionic bonding. While technically not an intermolecular force, the strong electrostatic attractions between Na+ and Cl- ions dictate its properties. There are very weak van der Waals forces as well, but these are insignificant compared to the ionic forces. The behavior of NaCl is essentially all about the strong electrostatic interactions between the ions in the crystal lattice.
Why are NaCl intermolecular forces stronger than those in, say, water?
The interactions in water are hydrogen bonds, which are strong intermolecular forces, but still weaker than the ionic bonds in NaCl. NaCl intermolecular forces (or rather, ionic bonds) involve full charges (+1 and -1) compared to the partial charges in water’s hydrogen bonds. This difference in charge magnitude is what makes the forces within NaCl so much more powerful.
How does the strength of nacl intermolecular forces affect its melting point?
The high melting point of NaCl (801 °C) is a direct result of the strong forces (ionic bonds) holding the Na+ and Cl- ions together. Significant energy is required to overcome these electrostatic attractions and allow the ions to move freely, transitioning from solid to liquid. Weak nacl intermolecular forces would translate to a much lower melting point.
Are nacl intermolecular forces affected by temperature?
Yes, temperature influences the strength of the ionic interactions in NaCl. Higher temperatures increase the kinetic energy of the ions, making them vibrate more vigorously. While the fundamental strength of the electrostatic attraction remains, the increased motion can slightly weaken the overall stability of the crystal lattice, eventually leading to melting if the temperature is sufficiently high.
So there you have it! Hopefully, you’ve now got a solid grasp on nacl intermolecular forces. Keep experimenting, keep questioning, and keep exploring the amazing world of chemistry!