Microbiology laboratories rely heavily on microbial media types for cultivating microorganisms, and understanding their diverse range is crucial for accurate results. The composition of these media directly impacts microbial growth, making selection a critical decision. Nutrient availability within a given media formulation determines which organisms can thrive, and consequently, the types of analyses possible. Oxoid, a prominent manufacturer, offers a wide array of microbial media types designed to support various research and diagnostic applications. Therefore, carefully evaluating the characteristics of each media, especially concerning its suitability for specific microorganisms and experimental goals, ensures successful experiments, contributing to advancements in disease diagnosis and scientific understanding.
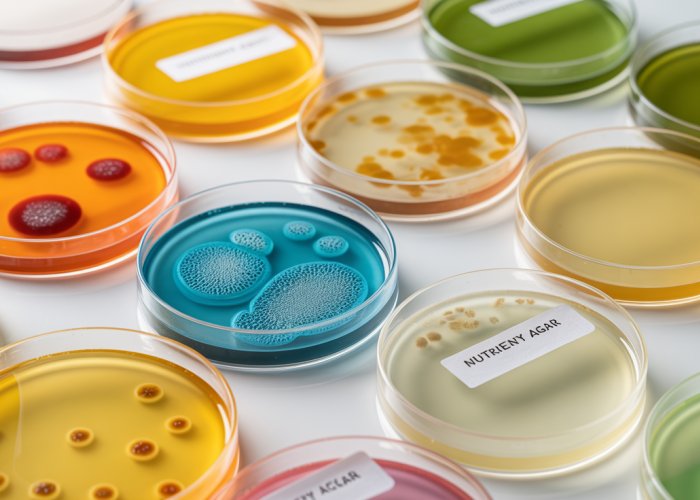
Microbial media stands as a fundamental pillar in the vast and intricate world of microbiology. It serves as the very foundation upon which we cultivate and study microorganisms. These carefully crafted formulations provide the necessary nutrients and environmental conditions for bacteria, fungi, viruses, and other microbes to thrive, allowing us to observe their behavior, understand their characteristics, and harness their potential.
The choice of the correct media type is paramount. It’s not merely a matter of providing sustenance; it’s about creating a selective environment tailored to specific experimental needs and research objectives.
Defining Microbial Media: A Foundation for Life
At its core, microbial media is a specially formulated substance designed to support the growth of microorganisms. It acts as an artificial ecosystem, providing the essential building blocks and energy sources that microbes need to replicate and flourish.
Without this controlled environment, the study and manipulation of microorganisms would be virtually impossible.
The Significance of Microbial Media
The significance of microbial media extends far beyond the laboratory bench. It plays a vital role in diverse fields, including:
-
Medical diagnostics: Identifying pathogens and determining antibiotic sensitivities.
-
Pharmaceuticals: Developing and testing new drugs.
-
Food safety: Detecting and preventing foodborne illnesses.
-
Environmental science: Studying microbial communities in various ecosystems.
Supporting Microbial Growth: A Diverse Ecosystem
Microbial media comes in a vast array of formulations, each designed to cater to the specific nutritional requirements of different types of microorganisms.
Whether it’s cultivating fastidious bacteria, growing robust fungi, or even propagating viruses within host cells, the right media provides the necessary building blocks for growth.
This includes essential elements like carbon, nitrogen, vitamins, minerals, and other growth factors. These elements are crucial for metabolic processes and cellular replication.
Selecting the Right Media: A Critical Decision
The selection of appropriate microbial media is a critical decision that can significantly impact the outcome of any microbiological experiment.
Different microorganisms have different nutritional needs. Some require a rich and complex media, while others thrive on simpler, more defined formulations.
The specific application also plays a key role. For example, a selective media may be used to isolate a particular type of bacteria from a mixed culture, while a differential media may be used to distinguish between different species based on their metabolic activities.
Careful consideration of these factors is essential for ensuring successful microbial cultivation and obtaining meaningful results.
Microbial media comes in a vast array of formulations, each designed to cater to the specific nutritional requirements of different types of microorganisms. Whether it’s cultivating fastidious bacteria, growing robust fungi, or even propagating viruses within host cells, the right medium is paramount. Let’s delve deeper into what exactly constitutes microbial media and explore its diverse components.
Defining Microbial Media: The Foundation of Cultivation
At its essence, microbial media is a scientifically engineered formulation specifically designed to foster the growth and propagation of microorganisms in a controlled laboratory setting. It serves as an artificial nutritional ecosystem.
This provides the essential elements necessary for microbes to thrive, multiply, and exhibit their inherent biological processes.
Essential Components of Microbial Media
Microbial media isn’t just a random concoction; it’s a precisely crafted blend of essential components, each playing a crucial role in supporting microbial life:
-
Nutrient Sources: These form the energetic and material bedrock for microbial growth. Sources can range from simple sugars like glucose to more complex mixtures found in substances like Nutrient Broth. Nutrient broth typically contains peptones (partially digested proteins) and beef extract, providing a rich source of amino acids, peptides, vitamins, and minerals that microbes can readily utilize.
-
Solidifying Agent: While not always necessary, solidifying agents are used to create solid or semi-solid media. Agar, a polysaccharide derived from seaweed, is the most commonly used solidifying agent due to its unique properties. Agar is inert, meaning it doesn’t react with the nutrients in the media, and it remains solid at typical incubation temperatures.
-
Growth Factors: Certain microorganisms, especially those with complex nutritional requirements, may require specific growth factors to thrive. These can include vitamins, amino acids, purines, pyrimidines, or other organic molecules that the microbe cannot synthesize on its own. The inclusion of specific growth factors can dramatically improve the cultivation of fastidious organisms.
-
Buffering Agents: Maintaining a stable pH is crucial for microbial growth. Buffering agents, such as phosphates, are often added to media to resist changes in pH caused by microbial metabolism. This ensures that the media remains within the optimal pH range for the target microorganism.
-
Selective Agents: These are added to selective media to inhibit the growth of unwanted microorganisms while allowing the growth of the desired ones. Selective agents can include antibiotics, dyes, or specific chemicals that are toxic to certain microbes but tolerated by others.
Physical States of Microbial Media
Microbial media exists in three primary physical states, each suited for different applications:
-
Solid Media: Solid media, typically created by adding agar to a nutrient broth, provides a firm surface for microbial growth. This is ideal for isolating individual colonies, observing colony morphology, and performing various microbiological tests. Agar plates are a classic example of solid media.
-
Liquid Media: Liquid media, also known as broth, lacks a solidifying agent. It’s used for growing large quantities of microorganisms, studying microbial metabolism, and performing biochemical assays. Nutrient Broth is a widely used liquid medium.
-
Semi-Solid Media: Semi-solid media contains a lower concentration of agar than solid media, resulting in a soft, gel-like consistency. It’s often used to determine microbial motility or to cultivate microaerophilic organisms (those that require low oxygen levels). Motility Agar is a common example.
A Comprehensive Guide to Microbial Media Types
Microbial media are not created equal.
Instead, they are meticulously formulated to serve diverse purposes in microbiology.
Each category possesses unique characteristics that make it suitable for specific applications.
Let’s explore the landscape of microbial media types, focusing on their composition, uses, and defining examples.
A. Nutrient Agar: The All-Purpose Workhorse
Nutrient Agar is a widely utilized general-purpose medium in microbiology laboratories.
Its composition typically includes peptone, beef extract, and agar.
These components provide a broad spectrum of nutrients suitable for supporting the growth of many non-fastidious microorganisms.
Nutrient Agar’s versatility stems from its simple yet effective formulation.
It supports a wide range of bacteria and some fungi.
This makes it ideal for introductory microbiology courses, basic research, and maintaining stock cultures.
Nutrient Agar allows for observable colony morphology, making it a great entry point for understanding the fundamentals of microbial growth.
B. Selective Media: Isolating Specific Microbes
Selective Media is engineered to inhibit the growth of certain microorganisms while allowing others to thrive.
This selectivity is achieved by incorporating specific inhibitors, antibiotics, or other compounds into the media’s formulation.
The underlying principle is to create an environment where only the desired microorganisms can effectively compete for resources.
MacConkey Agar is a prime example of selective media.
It contains bile salts and crystal violet, which inhibit the growth of Gram-positive bacteria, making it selective for Gram-negative bacteria.
Furthermore, MacConkey Agar differentiates between lactose fermenters and non-lactose fermenters through the inclusion of lactose and a pH indicator.
Selective media are indispensable in isolating specific types of microorganisms from mixed populations, such as clinical samples or environmental specimens.
This is vital for identifying pathogens and studying microbial communities.
C. Differential Media: Distinguishing Microbial Species
Differential Media enables the visual differentiation of microorganisms based on their metabolic activities.
This is achieved by incorporating specific substrates or indicators into the media that react differently depending on the microbe’s enzymatic capabilities.
Blood Agar is a classic example of differential media.
It contains mammalian blood cells.
This allows for the detection of hemolysis, or the breakdown of red blood cells, by bacterial colonies.
Different patterns of hemolysis (alpha, beta, or gamma) indicate different species.
As mentioned earlier, MacConkey Agar also serves as a differential medium, differentiating between lactose-fermenting and non-lactose-fermenting bacteria.
Lactose fermenters produce acid, which lowers the pH and turns the pH indicator pink or red.
Non-lactose fermenters do not produce acid, and their colonies remain colorless or transparent.
Differential media provides valuable information about the metabolic properties of microorganisms.
It aids in their identification and classification based on observable reactions.
D. Enrichment Media: Boosting Microbial Populations
Enrichment Media promotes the growth of a particular microorganism without necessarily inhibiting the growth of others.
It typically contains specific nutrients or growth factors that give the desired microorganism a competitive advantage.
The principle behind enrichment media is to increase the population of the target microorganism to detectable levels.
This is particularly useful when the desired microbe is present in low numbers within a complex sample.
Enrichment Media creates conditions that favor the growth of the target organism.
This makes it easier to isolate and identify using other methods.
E. Defined Media (Synthetic Media): Precision in Composition
Defined Media, also known as Synthetic Media, is characterized by its precisely known chemical composition.
Every ingredient, along with its exact concentration, is defined.
This allows for meticulous control over the nutrients available to the microorganisms.
The advantage of using Defined Media lies in its reproducibility and the ability to study the specific nutritional requirements of microorganisms.
Researchers can manipulate the medium to study the effect of individual nutrients.
The disadvantage is that it can be more complex and expensive to prepare compared to Complex Media.
Defined Media finds applications in research settings where precise control over nutrient availability is essential, such as metabolic studies and genetic experiments.
F. Complex Media: Rich and Undefined
Complex Media contains ingredients of variable chemical composition, such as peptones, yeast extract, or beef extract.
The exact composition of these ingredients is not precisely known, hence the term "complex".
Nutrient Broth is a common example of Complex Media.
It offers a rich source of amino acids, peptides, vitamins, and minerals that support the growth of a wide range of microorganisms.
The advantage of using Complex Media is its simplicity and its ability to support the growth of many different microorganisms.
The disadvantage is that the variable composition makes it difficult to control the precise nutrient availability.
Complex Media is suitable for routine cultivation, general microbiological assays, and growing microorganisms for various applications.
G. Specialized Media: Tailored for Specific Needs
Specialized Media refers to media designed for specific purposes or to cultivate microorganisms with unique growth requirements.
Thioglycolate Broth is an example of specialized media used to culture anaerobic bacteria.
It contains sodium thioglycolate, which reduces oxygen in the medium, creating an anaerobic environment.
It also contains a small amount of agar to increase viscosity and inhibit oxygen diffusion.
A redox indicator is included to visualize the oxygen gradient.
Specialized media are vital in isolating and studying microorganisms with unique metabolic capabilities or specific environmental requirements.
Selective media is a powerful tool, but it’s only one piece of the puzzle. The world of microbial media is vast and varied, each type offering unique advantages for specific applications. Now, with a grasp on the different categories of media, let’s explore the key factors that guide the selection of the most appropriate media for your specific needs.
Key Factors in Microbial Media Selection
Choosing the right microbial medium is not a trivial task. It’s a critical decision that directly impacts the success of any microbiological experiment or process. Three primary considerations guide this choice: the specific microorganism being cultured, the objective of the culture, and the available resources.
Matching Media to Microorganism
The cardinal rule of media selection is that the medium must be compatible with the microorganism you intend to cultivate. Different microbes possess vastly different nutritional requirements.
Some are fastidious, demanding a complex blend of pre-formed organic compounds, while others are non-fastidious and can thrive on simpler nutrient sources.
Nutritional Needs
Fungi, for instance, typically require a slightly acidic pH and a carbon source like glucose or maltose.
Bacteria exhibit a far wider range of nutritional preferences, with some requiring specific amino acids, vitamins, or trace elements.
Furthermore, some microorganisms are obligate aerobes, requiring oxygen for growth, while others are obligate anaerobes and are killed by its presence. This dictates whether the media should be prepared and incubated under aerobic or anaerobic conditions, and even specialized media such as Thioglycolate Broth.
Ignoring these basic requirements will inevitably lead to poor growth, inaccurate results, or even complete failure of the culture.
Aligning Media with Research Objectives
The purpose for which you are culturing the microorganism is another crucial determinant in media selection. Are you trying to isolate a specific organism from a mixed population? Are you trying to identify a particular species based on its metabolic characteristics? Or are you trying to quantify the number of organisms in a sample?
Isolation, Identification, and Enumeration
For isolation, selective media is invaluable as mentioned before.
For identification, differential media comes to the fore, enabling you to distinguish between different species based on their visual characteristics. For example, Blood Agar can differentiate bacteria based on their hemolytic activity.
For enumeration, a general-purpose medium like Nutrient Agar, combined with serial dilution and plate counting techniques, may suffice.
Resource Availability and Practical Considerations
Practical considerations also play a significant role in media selection. High-end selective and differential media can sometimes be expensive, especially when testing multiple environmental samples or processing samples on a large scale.
Cost, Equipment, and Expertise
Cost is always a factor, especially in resource-limited settings. The availability of specialized equipment, such as anaerobic chambers or autoclaves, may also limit your options. Finally, the expertise of the personnel involved is another crucial factor. Some media require more careful preparation and handling than others, and it’s important to choose a medium that can be reliably prepared and used by the available staff.
Selective media is a powerful tool, but it’s only one piece of the puzzle. The world of microbial media is vast and varied, each type offering unique advantages for specific applications. Now, with a grasp on the different categories of media, let’s explore the key factors that guide the selection of the most appropriate media for your specific needs.
Preparing and Utilizing Microbial Media: A Practical Guide
The right microbial medium, carefully chosen, is only the starting point. To ensure successful and reliable results, proper preparation, sterilization, and handling are paramount. These steps eliminate unwanted contaminants and maintain the media’s integrity, paving the way for accurate and meaningful observations.
Step-by-Step Guide to Preparing Nutrient Agar
Nutrient Agar remains a staple in many microbiology labs, serving as a versatile platform for cultivating a wide array of microorganisms. Its preparation is straightforward, yet adherence to specific steps is critical.
-
Calculating Requirements: Begin by determining the volume of Nutrient Agar needed based on the number of plates or tubes to be prepared. Consult the manufacturer’s instructions for the correct agar-to-water ratio, typically around 23 grams of dehydrated Nutrient Agar powder per liter of distilled water.
-
Mixing the Ingredients: In a flask of appropriate size, dissolve the calculated amount of Nutrient Agar powder in distilled water. Use a magnetic stirrer or gentle swirling to ensure uniform distribution and prevent clumping.
-
Heating and Dissolving: Heat the mixture on a hot plate with a stirring function or using a microwave in short bursts, stirring in between. Bring the mixture to a boil, ensuring the agar is completely dissolved. The solution should appear clear and free of any particulate matter.
-
Sterilization: Sterilize the dissolved Nutrient Agar by autoclaving at 121°C (250°F) for 15 minutes. This step is crucial for eliminating any contaminating microorganisms present in the media or glassware.
-
Cooling and Pouring: Allow the autoclaved Nutrient Agar to cool to around 45-50°C (113-122°F) before pouring. At this temperature, the agar remains liquid but is cool enough to prevent excessive condensation on the petri dish lids.
-
Pouring Plates: Under sterile conditions (ideally within a laminar flow hood), carefully pour the molten Nutrient Agar into sterile petri dishes. Fill each dish to a depth of approximately 4 mm, ensuring a smooth, even surface.
-
Solidification and Storage: Allow the agar to solidify completely at room temperature. Once solidified, invert the plates and store them in a refrigerator at 2-8°C (35-46°F) until needed.
Mastering Sterilization Techniques: Eradicating Contamination
Sterilization is the cornerstone of aseptic technique and critical for preventing unwanted microbial growth in culture media. Several methods are commonly employed, each with its advantages and limitations.
-
Autoclaving: Autoclaving, as previously mentioned, uses high-pressure steam to achieve sterilization. It is the preferred method for sterilizing Nutrient Agar and other heat-stable media. The combination of high temperature and pressure effectively kills bacteria, fungi, viruses, and spores.
-
Filter Sterilization: For heat-sensitive media components, filter sterilization offers a viable alternative. This technique involves passing the liquid medium through a sterile filter with a pore size small enough to trap microorganisms (typically 0.22 μm).
-
Dry Heat Sterilization: Dry heat sterilization, typically performed in an oven at high temperatures (e.g., 160-170°C for 2 hours), is suitable for sterilizing glassware and other heat-resistant materials. However, it is not appropriate for sterilizing most culture media due to the risk of degradation.
-
UV Radiation: Ultraviolet (UV) radiation can be used to sterilize surfaces and air within a biological safety cabinet. However, it has limited penetrating power and is not effective for sterilizing liquids or opaque materials.
Proper Storage and Handling: Maintaining Media Integrity
Once prepared and sterilized, microbial media must be stored and handled correctly to maintain its integrity and prevent contamination.
-
Storage Conditions: Prepared agar plates should be stored inverted in a refrigerator at 2-8°C (35-46°F). This minimizes moisture condensation on the agar surface, which can promote the growth of contaminants. Broth media should also be refrigerated to slow down any potential microbial growth.
-
Shelf Life: While the specific shelf life varies depending on the media type and storage conditions, it’s generally recommended to use prepared media within a few weeks. Check for signs of contamination (e.g., discoloration, cloudiness, visible colonies) before use.
-
Aseptic Handling: When handling sterile media, always practice aseptic technique. Work in a clean environment, preferably within a laminar flow hood. Use sterile pipettes, loops, and other instruments. Avoid touching the sterile surfaces of the media or containers.
-
Disposal: Used microbial media should be properly disposed of according to institutional guidelines. Autoclaving is a common method for sterilizing contaminated media before disposal.
Frequently Asked Questions About Microbial Media Types
Here are some common questions about microbial media types to help you choose the best one for your needs.
What’s the main difference between defined and complex microbial media?
Defined media have precisely known chemical compositions, which is useful for research where you need tight control. Complex media contain ingredients with unknown composition, like yeast extract, making them easier and cheaper to prepare, and suitable for general cultivation. The choice depends on your experiment’s precision needs.
When should I use selective microbial media?
Selective media are designed to inhibit the growth of some microorganisms while allowing others to thrive. This is particularly helpful when you need to isolate a specific type of microbe from a mixed population. For example, to isolate a specific bacteria from a soil sample.
What are differential microbial media used for?
Differential media allow you to distinguish between different types of microorganisms based on their observable characteristics. This often involves color changes or other visual indicators that result from specific metabolic activities. An example of this is blood agar, which can differentiate bacteria based on their ability to lyse red blood cells.
How do I choose the right microbial media type for my experiment?
Consider the specific needs of the microorganism you’re trying to grow. Think about nutritional requirements, desired selectivity, and whether you need to differentiate between types of microbes. Consider factors like cost and ease of preparation, before selecting the appropriate microbial media types.
Hopefully, this gives you a better handle on microbial media types! Choosing the right one can seem tricky, but now you’ve got a solid starting point. Best of luck with your experiments and happy culturing!