Understanding krypton atomic structure involves examining its arrangement of electrons and protons, a concept central to atomic physics. The noble gas’s properties are directly attributable to its electronic configuration. Linus Pauling, a pioneer in chemical bonding, contributed significantly to our understanding of electron distribution in atoms, including insights applicable to krypton research facilities, such as those exploring its uses in lighting.
Krypton, a name derived from the Greek word kryptos meaning "the hidden one," is indeed an element that often flies under the radar. As a noble gas residing in Group 18 of the periodic table, it shares the spotlight with more commonly recognized elements like helium and neon.
Yet, Krypton possesses a unique allure, stemming from its distinctive atomic properties and surprisingly diverse applications.
Krypton: A Noble Gas with Notable Properties
Noble gases, also known as inert gases, are characterized by their exceptional stability and reluctance to participate in chemical reactions. This stems from their full outer electron shells, a configuration that renders them exceptionally stable. Krypton, with its atomic number of 36, exemplifies this characteristic, exhibiting a complete octet of electrons in its outermost shell.
Real-World Applications of Krypton
Despite its inherent inertness, Krypton has found its niche in various technological applications.
One of its most well-known uses is in lighting. Krypton-filled lamps are prized for their bright, white light and long lifespan. This makes them ideal for applications ranging from airport runway lighting to high-performance flashlights.
Krypton also plays a crucial role in certain types of lasers.
Specifically, krypton fluoride (KrF) excimer lasers are used in microlithography, a critical process in the manufacturing of semiconductors. These lasers produce high-energy ultraviolet light that allows for the creation of incredibly fine patterns on silicon wafers, enabling the production of advanced microchips.
Beyond lighting and lasers, Krypton finds use in specialized areas such as:
-
Photography: For high-speed photography, capturing fast-moving objects with exceptional clarity.
-
Medical imaging: In some specialized imaging techniques, leveraging its properties for enhanced visualization.
Decoding the Atom: A Visual Journey into Krypton’s Structure
This article embarks on a journey to demystify Krypton, offering a comprehensive and visually-rich exploration of its atomic structure.
We will delve into the arrangement of its protons, neutrons, and electrons. Further, the visual representation will clarify how these subatomic particles interact to define Krypton’s unique identity and behavior.
Our aim is to provide a clear and accessible understanding of Krypton’s atomic architecture, illuminating its significance in the broader context of chemistry and the natural world.
Krypton, while exceptional, is built upon the same foundational principles that govern all matter in the universe. Understanding its atomic structure necessitates a firm grasp of the basic building blocks that constitute every element.
Atomic Structure: The Building Blocks Explained
At the heart of all matter lies the atom, the smallest unit of an element that retains the chemical properties of that element. Understanding the structure of an atom is fundamental to understanding the behavior of elements, including Krypton. Atoms are composed of three primary subatomic particles: protons, neutrons, and electrons.
Protons, Neutrons, and Electrons: The Subatomic Trinity
Protons, found within the atom’s nucleus, carry a positive electrical charge.
Neutrons, also residing in the nucleus, are electrically neutral, possessing no charge.
Electrons, far smaller than protons and neutrons, orbit the nucleus in a cloud and carry a negative charge.
The balance between these charges is crucial to the atom’s overall stability.
Charge and Location: A Matter of Arrangement
The location and charge of each subatomic particle are critical to understanding atomic behavior.
The nucleus, the atom’s central core, houses the protons and neutrons, accounting for almost all of the atom’s mass.
The positive charge of the protons binds the negatively charged electrons in orbit around the nucleus, forming a stable and structured atom.
Electrons occupy specific energy levels or shells, dictating their distance from the nucleus.
Atomic Number: The Element’s Identity Card
The atomic number is the defining characteristic of an element. It represents the number of protons found in the nucleus of every atom of that element.
This number is unique for each element and serves as its "identity card" on the periodic table.
For example, an element with 6 protons is always carbon, and an element with 79 protons is always gold.
Changing the number of protons transforms the atom into a different element altogether.
Mass Number and Isotopes: Variations on a Theme
The mass number represents the total number of protons and neutrons in an atom’s nucleus.
While the number of protons is constant for a given element, the number of neutrons can vary.
Atoms of the same element with different numbers of neutrons are called isotopes.
For instance, carbon-12 (12C) has 6 protons and 6 neutrons, while carbon-14 (14C) has 6 protons and 8 neutrons.
Isotopes of an element exhibit the same chemical properties but differ slightly in mass and nuclear stability.
Krypton, while exceptional, is built upon the same foundational principles that govern all matter in the universe. Understanding its atomic structure necessitates a firm grasp of the basic building blocks that constitute every element.
Now, let’s delve deeper into what makes Krypton unique. Specifically, we will examine its atomic number and how it relates to its mass number, paving the way to understanding the fascinating concept of isotopes.
Krypton’s Identity: Atomic and Mass Numbers
Every element possesses a unique identity card, a number that firmly defines it within the grand scheme of the periodic table. For Krypton, this identity is crystal clear: 36.
The Significance of Atomic Number 36
Krypton’s atomic number is 36. This number is not arbitrary; it is the cornerstone of Krypton’s identity.
It signifies that every Krypton atom, without exception, contains 36 protons within its nucleus.
This proton count is what distinguishes Krypton from all other elements. Change the number of protons, and you fundamentally change the element itself.
Deciphering the Mass Number
While the atomic number firmly defines the element, the mass number offers further insight into the composition of a specific atom.
The mass number represents the total count of protons and neutrons residing within the nucleus. To approximate Krypton’s most common mass number, you might be tempted to simply look at the periodic table, where an average atomic mass of around 83.8 u (atomic mass units) is listed.
However, since mass number refers to a specific isotope (more on that soon), we need to think about the most abundant isotopes to grasp the common range.
The mass number isn’t a fixed value for Krypton because of the existence of isotopes.
Isotopes: Variations on a Theme
The concept of isotopes introduces a fascinating twist to our understanding of atomic structure. Isotopes are atoms of the same element (same number of protons) that possess different numbers of neutrons.
This difference in neutron count results in variations in the mass number. Krypton, like many elements, exists in nature as a mixture of several isotopes.
For example, Krypton-84 (84Kr) possesses 36 protons and 48 neutrons (84 – 36 = 48). Another common isotope is Krypton-86 (86Kr), which contains 36 protons and 50 neutrons.
The existence of isotopes explains why the atomic mass listed on the periodic table is a decimal number, representing the weighted average of the masses of all naturally occurring isotopes. Common Krypton isotopes include:
- Krypton-78 (78Kr)
- Krypton-80 (80Kr)
- Krypton-82 (82Kr)
- Krypton-83 (83Kr)
- Krypton-84 (84Kr)
- Krypton-86 (86Kr)
These isotopes contribute differently to the overall average atomic mass of Krypton.
While the atomic and mass numbers provide a snapshot of the nucleus, they don’t tell the whole story. The behavior of an element, its interactions with others, is largely determined by the arrangement of its electrons. This arrangement, known as the electron configuration, is the key to unlocking Krypton’s chemical personality.
Electron Configuration: Arranging the Electrons Around Krypton
Electron configuration is a fundamental concept in chemistry.
It describes the specific arrangement of electrons within an atom. This arrangement dictates an element’s chemical properties. It determines how it will interact with other atoms.
Understanding Electron Configuration
Imagine an atom as a multi-story building, with each floor representing a different energy level.
Electrons, the atom’s residents, occupy specific rooms (orbitals) within these floors.
Electron configuration is simply a way of listing which rooms are occupied, and how many electrons are in each.
This arrangement isn’t random. It follows specific rules dictated by quantum mechanics.
Krypton’s Electron Configuration: A Detailed Look
Krypton, with its 36 electrons, has a particular electron configuration: 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁶.
This notation might seem cryptic at first. But it’s a straightforward way of representing the electron arrangement.
Let’s break it down:
-
The numbers (1, 2, 3, 4) represent the energy levels or electron shells. Higher numbers indicate greater distance from the nucleus and higher energy.
-
The letters (s, p, d) represent orbitals, which are regions within each energy level where electrons are likely to be found. ‘s’ orbitals are spherical, ‘p’ orbitals are dumbbell-shaped, and ‘d’ orbitals have more complex shapes.
-
The superscripts (², ⁶, ¹⁰) indicate the number of electrons occupying each orbital.
So, "1s²" means that the first energy level (n=1) has its ‘s’ orbital filled with two electrons.
"2p⁶" means that the second energy level (n=2) has its ‘p’ orbitals filled with six electrons.
Decoding the Notation
Each orbital can hold a maximum number of electrons. An ‘s’ orbital can hold up to 2 electrons, a ‘p’ orbital can hold up to 6 electrons, and a ‘d’ orbital can hold up to 10 electrons.
Krypton’s configuration shows that its electrons completely fill the 1s, 2s, 2p, 3s, 3p, 4s, 3d, and 4p orbitals.
This complete filling is crucial to understanding its noble gas behavior.
Visualizing Electron Configuration: The Orbital Diagram
While the written configuration is informative, a visual representation can provide a more intuitive understanding. An orbital diagram uses boxes or lines to represent individual orbitals and arrows to represent electrons.
Each box can hold a maximum of two electrons. These are represented by upward and downward pointing arrows (to indicate opposite spin).
For Krypton, the orbital diagram would show all orbitals up to 4p being fully occupied. This provides a visual confirmation of its stable electron arrangement.
While the detailed electron configuration notation provides a precise account of electron arrangement, it can be abstract. A more intuitive way to understand electron placement involves visualizing electron shells, which offer a simplified yet powerful model of how electrons orbit the nucleus.
Electron Shells and Energy Levels: Visualizing Electron Placement
Electron shells, sometimes referred to as energy levels, provide a simplified model for visualizing electron arrangement around the nucleus. This model, while less precise than the full electron configuration, offers a clear and intuitive understanding of electron distribution and energy quantization.
Understanding Electron Shells
Imagine the atom as an onion, with layers surrounding the central nucleus. These layers are the electron shells, each designated by a letter: K, L, M, N, and so on, moving outwards from the nucleus.
-
The K shell is closest to the nucleus and corresponds to the lowest energy level (n=1).
-
The L shell is the second layer (n=2).
-
The M shell is the third (n=3), and so on.
Each shell can hold a maximum number of electrons, dictated by the formula 2n², where n is the shell number.
-
The K shell (n=1) can hold up to 2 electrons.
-
The L shell (n=2) can hold up to 8 electrons.
-
The M shell (n=3) can hold up to 18 electrons.
-
The N shell (n=4) can hold up to 32 electrons.
Krypton’s Electron Distribution
Krypton, with its 36 electrons, demonstrates how these shells are filled. The electrons arrange themselves to minimize their energy, filling the innermost shells first before moving to the outer ones.
-
The K shell (n=1) accommodates 2 electrons.
-
The L shell (n=2) accommodates 8 electrons.
-
The M shell (n=3) accommodates 18 electrons.
-
This leaves 8 electrons to occupy the N shell (n=4).
Therefore, Krypton’s electron distribution across its shells is 2, 8, 18, 8. This arrangement contributes significantly to its chemical inertness, as will be explained in the following section.
Energy Levels and Quantization
Electron shells are directly linked to energy levels. Electrons in the K shell possess the lowest energy, while those in the outer shells possess progressively higher energy. This isn’t a continuous spectrum of energy but rather a quantized one, meaning electrons can only exist at specific energy levels corresponding to the shells.
Electrons can transition between energy levels by absorbing or emitting energy in the form of photons.
The amount of energy required for a transition between two shells is specific and corresponds to a particular wavelength of light. This quantization of energy is a fundamental principle of quantum mechanics and governs the behavior of electrons within the atom.
While visualizing electron shells provides a simplified picture of electron arrangement, it’s crucial to understand how these arrangements influence an element’s behavior. This leads us to the concept of valence electrons and how they explain Krypton’s noble gas status.
Valence Electrons and Inertness: The Key to Krypton’s Noble Nature
Defining Valence Electrons
Valence electrons are the electrons residing in the outermost electron shell of an atom.
These are the electrons that participate in chemical bonding.
They determine the atom’s chemical properties and how it interacts with other atoms.
Atoms "want" to achieve a stable electron configuration, typically resembling that of a noble gas.
Krypton’s Full Outer Shell: Eight is Enough
Krypton, with its atomic number of 36, has the electron configuration 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁶.
Notice the outermost shell, the fourth shell, contains the 4s² and 4p⁶ orbitals.
Adding the electrons in these orbitals together, we find that Krypton possesses eight valence electrons.
This is a full outer shell, also known as an octet.
The Octet Rule and Chemical Inertness
The octet rule dictates that atoms tend to gain, lose, or share electrons in order to achieve a full outer shell of eight electrons, mimicking the stable configuration of noble gases.
Because Krypton already has a full outer shell, it has minimal tendency to form chemical bonds.
This is why Krypton is considered chemically inert or unreactive.
It doesn’t readily participate in chemical reactions because it’s already in a stable, low-energy state.
Exceptions to the Rule: Krypton Compounds
While Krypton is generally considered inert, it’s not entirely unreactive.
Under extreme conditions, Krypton can form compounds with highly electronegative elements like fluorine and oxygen.
The most well-known example is Krypton difluoride (KrF₂).
This compound is formed by passing an electrical discharge through a mixture of Krypton and fluorine gases at very low temperatures.
Other Krypton compounds have also been synthesized, but they are generally unstable and require special conditions.
The formation of these compounds demonstrates that even noble gases aren’t completely inert.
It highlights the fact that chemical reactivity is a spectrum, and even elements with full outer shells can participate in bonding under the right circumstances.
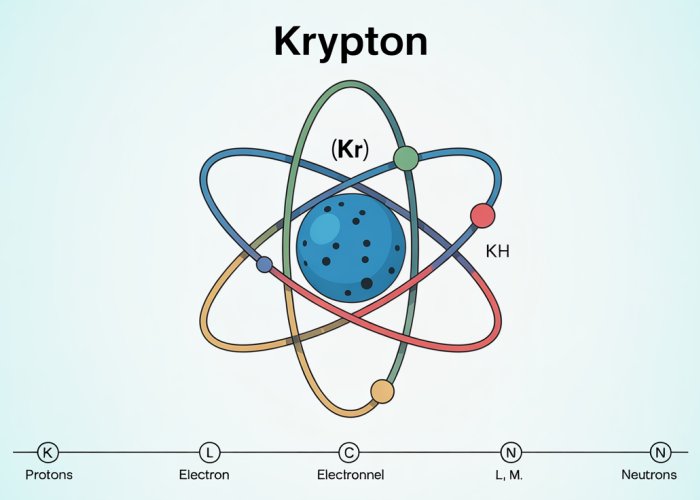
Visualizing Krypton’s Atom: Diagrams and Models
Understanding Krypton’s atomic structure is significantly enhanced through visual aids. Diagrams and models provide a concrete representation of abstract concepts, allowing for a more intuitive grasp of the arrangement of subatomic particles.
The Nucleus: A Central Hub
The atomic nucleus, the atom’s core, is where nearly all of its mass resides. A clear diagram should depict this nucleus, emphasizing its composition of protons and neutrons.
Differentiating Protons and Neutrons
Employing distinct colors or shading is crucial. One color can represent protons (positively charged particles), while another represents neutrons (electrically neutral particles).
This visual distinction immediately clarifies the nuclear composition and reinforces the concept that the atomic number (number of protons) defines the element.
Labeling for Clarity
Each proton and neutron within the nucleus should be clearly labeled. This meticulous labeling avoids ambiguity and allows the reader to easily identify each component.
Electron Shells: Visualizing Electron Orbitals
Beyond the nucleus, the arrangement of electrons plays a pivotal role in determining Krypton’s chemical properties. A diagram showcasing the electron shells is essential.
Depicting Electron Configuration
This diagram should accurately represent Krypton’s electron configuration (1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁶). Each shell (K, L, M, N) should be visibly distinct.
The K shell (closest to the nucleus) holds a maximum of 2 electrons. The L shell holds 8, the M shell 18, and the N shell, in Krypton’s case, holds 8 valence electrons.
Illustrating Electron Placement
Electrons should be placed within their respective shells according to the electron configuration. This visual representation clearly demonstrates the concept of quantized energy levels.
Distinguishing Electrons Visually
Similar to the nucleus diagram, using a different color or shading for electrons is highly recommended. This visual cue further separates electrons from protons and neutrons, aiding in comprehension.
Complete and Clear Labeling
Labeling each electron shell (K, L, M, N) and the number of electrons within each shell is paramount. This ensures that the reader can easily correlate the diagram with the written electron configuration.
By meticulously labeling all components and using clear visual distinctions, diagrams of Krypton’s atom transform abstract concepts into tangible representations, greatly improving understanding of its atomic structure.
Krypton’s Place: The Periodic Table Context
Having explored the intricate details of Krypton’s atomic structure, it’s crucial to situate this element within the broader framework of the periodic table. By understanding its location and relationships with other elements, particularly its fellow noble gases, we gain a deeper appreciation for its unique properties and behavior.
Locating Krypton
Krypton resides in Group 18 (also known as Group 8A) of the periodic table, the distinguished family of noble gases. Its period, or row, is Period 4. This placement is not arbitrary; it directly reflects the element’s electron configuration and its resulting chemical properties.
The Noble Gas Family
The noble gases – Helium (He), Neon (Ne), Argon (Ar), Krypton (Kr), Xenon (Xe), and Radon (Rn) – share a characteristic feature: a complete outer electron shell. This fullness is what imparts their remarkable stability and reluctance to engage in chemical reactions under normal conditions.
Krypton’s position within this family is significant.
It sits between Argon and Xenon, exhibiting properties that are influenced by its neighbors.
As we move down the group, atomic size and complexity increase, leading to gradual shifts in physical and chemical behavior.
Trends in Atomic Properties
Several key atomic properties exhibit discernible trends as we descend the noble gas group. These trends offer insights into Krypton’s specific characteristics.
Atomic Size
Atomic size increases as we move down the group. This is because each successive element adds another electron shell, expanding the atom’s overall volume. Krypton, therefore, is larger than Argon but smaller than Xenon.
Ionization Energy
Ionization energy, the energy required to remove an electron from an atom, decreases as we move down the group. This is because the outermost electrons are farther from the nucleus and thus more easily removed. Krypton’s ionization energy is lower than that of Argon but higher than that of Xenon, reflecting its intermediate position.
Electronegativity
Electronegativity, the measure of an atom’s ability to attract electrons in a chemical bond, is generally considered to be very low for noble gases, owing to their stable electron configurations. While traditionally considered to be zero, heavier noble gases like Krypton can exhibit slight electronegativity under extreme conditions due to their larger, more polarizable electron clouds.
Krypton, while largely inert, can form compounds with highly electronegative elements like fluorine. This capability, though limited, underscores the influence of atomic size and electron cloud polarizability on chemical behavior.
Krypton’s Characteristics: Physical and Chemical Properties
Having established Krypton’s place on the periodic table, the next logical step is to examine its tangible characteristics. The physical and chemical properties of any element are not arbitrary; they are a direct consequence of its atomic structure and electron configuration. These properties dictate how Krypton interacts with the world and what applications it can serve.
Physical Attributes of Krypton
Krypton, at room temperature and standard pressure, exists as a colorless, odorless, and tasteless monatomic gas. This immediately highlights its noble gas nature; it doesn’t readily bond with itself or other elements to form diatomic molecules or complex structures. Its monatomic nature contributes to its relatively low density compared to many other gases.
Density and Phase Transitions
Krypton’s density, while seemingly inconsequential, plays a role in its industrial applications, particularly in specialized lighting. At its boiling point (-153.4 °C or -244.1 °F), liquid Krypton has a density significantly higher than its gaseous form. This drastic change in density is characteristic of phase transitions and reveals the relatively weak interatomic forces that exist in its liquid state.
The melting point of Krypton is even lower, at -157.2 °C (-251.0 °F). These extremely low melting and boiling points are indicative of the weak van der Waals forces – specifically London dispersion forces – that arise from temporary fluctuations in electron distribution.
Because Krypton atoms have a full outermost electron shell, they do not readily form strong bonds. This makes it difficult to bring Krypton molecules together.
Chemical Properties: Inertness and Reactivity
Krypton is renowned for its chemical inertness, a hallmark of the noble gas family. This inertness stems from its completely filled outer electron shell, containing eight valence electrons. This stable electron configuration satisfies the octet rule, making Krypton exceptionally resistant to forming chemical bonds with other elements under normal conditions.
The Illusion of Complete Inertness
However, the term "inert" is not entirely accurate. While Krypton is significantly less reactive than most other elements, it’s not completely devoid of chemical activity. Under extreme conditions, and with highly electronegative elements like fluorine and oxygen, Krypton can be coaxed into forming compounds.
The most notable example is Krypton difluoride (KrF₂), a colorless, crystalline solid that is thermodynamically unstable. The formation of KrF₂ requires extreme conditions, such as electrical discharge or photoionization, to provide the necessary energy to overcome Krypton’s inherent stability.
The formation of KrF₂ demonstrates that even the most inert elements can be induced to react. This occurs when sufficient energy is supplied. It also expanded our understanding of chemical bonding and the limits of the octet rule.
The limited number of known Krypton compounds underscores the challenges in forcing this noble gas to participate in chemical reactions. The conditions are demanding, and the resulting compounds are often unstable, highlighting the fundamental stability conferred by its filled electron shell.
Frequently Asked Questions: Krypton’s Atomic Structure
Here are some common questions about krypton’s atomic structure and how it relates to its properties.
What are the main components of a krypton atom?
A krypton atom consists of a nucleus containing 36 protons and a varying number of neutrons (depending on the isotope), surrounded by 36 electrons arranged in electron shells. The specific arrangement of these components defines the krypton atomic structure.
How many electron shells does krypton have, and how are they filled?
Krypton has four electron shells. The first shell holds 2 electrons, the second holds 8, the third holds 18, and the outermost (valence) shell holds 8 electrons. This complete outer shell is what makes krypton a noble gas, as a filled outer shell contributes to its inert nature due to the stable krypton atomic structure.
What determines the different isotopes of krypton?
The isotopes of krypton are determined by the number of neutrons in the nucleus. All krypton atoms have 36 protons, but the number of neutrons can vary, leading to different isotopes like krypton-84, krypton-86, etc. These variations don’t significantly alter the krypton atomic structure in terms of electron arrangement, but they do affect the atomic mass.
How does the electron configuration of krypton influence its reactivity?
Krypton’s electron configuration, specifically its full outermost electron shell, makes it exceptionally stable and unreactive. It rarely forms chemical bonds under normal conditions. The stable krypton atomic structure makes it a noble gas.
So, there you have it! Hopefully, this visual guide has shed some light on the fascinating world of krypton atomic structure. Go forth and explore the wonders of chemistry!