Understanding the kmno4 oxidation number is crucial for grasping redox reactions in chemistry. Potassium permanganate (KMnO4), a strong oxidizing agent, plays a significant role in titrations. The value of the oxidation number itself can be determined through established chemical principles. Furthermore, stoichiometry provides the framework for calculating the change in oxidation number during reactions involving KMnO4.
Potassium Permanganate, with its vibrant purple hue and chemical formula KMnO4, is a compound that extends far beyond the laboratory. From water treatment to medicinal applications, and even in art, KMnO4 plays a crucial role.
Its versatility stems from its powerful oxidizing properties, a characteristic intimately linked to the oxidation state of its constituent elements. Understanding these oxidation states unlocks a deeper understanding of KMnO4’s reactivity and applications.
This article serves as a straightforward guide.
Our goal is to demystify the process of determining the oxidation number of elements within Potassium Permanganate, with a specific focus on Manganese (Mn). We’ll break down the steps in a clear, accessible manner, making this seemingly complex topic easy to grasp.
What is Potassium Permanganate (KMnO4)?
Potassium Permanganate is an inorganic chemical compound comprising potassium ions (K+) and permanganate ions (MnO4−).
It appears as purple crystals or a crystalline powder and readily dissolves in water to form intensely purple solutions. This vibrant color is a key indicator of its presence and concentration.
Beyond its striking appearance, KMnO4 is a powerful oxidizing agent, meaning it readily accepts electrons from other substances, causing them to be oxidized. This property is fundamental to its diverse applications.
Common Uses of KMnO4
Water Treatment: KMnO4 is widely used to disinfect water. It oxidizes contaminants such as iron, manganese, hydrogen sulfide, and organic matter, improving water quality and taste.
Chemical Synthesis: In organic chemistry, KMnO4 serves as a reagent for various oxidation reactions. It can convert alcohols to aldehydes or ketones, and alkenes to diols or other oxygenated compounds.
Medical Applications: KMnO4 solutions are used as a disinfectant and antiseptic. They can treat skin conditions like fungal infections, eczema, and ulcers. However, it’s crucial to use diluted solutions to avoid irritation or burns.
Photography: Historically, KMnO4 was used in photographic processes as a toning agent.
Aquaculture: KMnO4 can control diseases and parasites in fish farms and aquariums.
Why Understanding Manganese’s Oxidation Number is Key
The oxidation number of Manganese (Mn) in KMnO4 is pivotal to understanding its behavior as an oxidizing agent. Manganese sits at the heart of the permanganate ion (MnO4-), and its ability to accept electrons during chemical reactions directly depends on its oxidation state.
By determining the oxidation number of Mn, we gain valuable insights into KMnO4’s reactivity and its capacity to participate in redox reactions. This knowledge is crucial for anyone working with KMnO4 in chemical, environmental, or medical contexts.
Potassium Permanganate’s remarkable ability to disinfect water and act as a potent reagent in chemical synthesis showcases its power. But to truly grasp how KMnO4 performs these diverse functions, one must understand the fundamental concept that drives its reactivity: oxidation numbers.
Decoding Oxidation Numbers: A Fundamental Concept
Oxidation numbers, also known as oxidation states, are a cornerstone of chemistry. They provide a way to track the flow of electrons during chemical reactions.
Understanding oxidation numbers is crucial for predicting how elements will interact and form compounds.
Defining Oxidation Number/Oxidation State
An oxidation number is essentially a charge assigned to an atom in a compound, assuming that all bonds are ionic.
It represents the hypothetical charge an atom would have if all bonds were completely ionic, meaning electrons were completely transferred.
It’s important to remember that oxidation numbers are a bookkeeping tool, not necessarily the actual charge on an atom.
They help us understand and predict how electrons are distributed in a molecule and how a chemical species will behave.
Key Rules for Assigning Oxidation Numbers
Assigning oxidation numbers follows a set of established rules. These rules are essential for correctly determining the oxidation state of elements in any given compound. Here are some key guidelines:
-
Elements in their elemental form: The oxidation number of an element in its pure, uncombined state is always zero. Examples include: $O2$, $N2$, $Cu$ (solid), etc.
-
Monatomic ions: The oxidation number of a monatomic ion is equal to its charge. For example, $Na^+$ has an oxidation number of +1, and $Cl^-$ has an oxidation number of -1.
-
Oxygen: Oxygen usually has an oxidation number of -2 in compounds. However, there are exceptions. In peroxides (like $H2O2$), oxygen has an oxidation number of -1.
-
Hydrogen: Hydrogen usually has an oxidation number of +1 in compounds. However, when bonded to a metal in a binary compound (metal hydrides such as $NaH$), hydrogen has an oxidation number of -1.
-
Fluorine: Fluorine is the most electronegative element and always has an oxidation number of -1 in its compounds.
-
Sum of oxidation numbers: The sum of the oxidation numbers in a neutral compound must equal zero. For polyatomic ions, the sum of the oxidation numbers must equal the charge of the ion.
Deducing Unknown Oxidation Numbers
One of the most powerful uses of oxidation numbers is determining the oxidation state of an element within a compound when its oxidation number is not immediately obvious.
This often involves working backward by applying the rules for known elements.
For example, in Potassium Permanganate ($KMnO_4$), we know that Potassium (K) typically has an oxidation number of +1 and Oxygen (O) has an oxidation number of -2.
Since the compound is neutral, the sum of all oxidation numbers must be zero.
This allows us to set up an algebraic equation and solve for the unknown oxidation number of Manganese (Mn), as we will explore in the next section.
Potassium Permanganate’s remarkable ability to disinfect water and act as a potent reagent in chemical synthesis showcases its power. But to truly grasp how KMnO4 performs these diverse functions, one must understand the fundamental concept that drives its reactivity: oxidation numbers.
KMnO4 Deconstructed: Identifying Its Components
Before we dive into the calculation, let’s dissect the KMnO4 molecule. We need to identify the individual elements that make up this compound. This is a crucial first step.
Potassium Permanganate, as its name suggests, is composed of three elements: Potassium (K), Manganese (Mn), and Oxygen (O). These elements are chemically bonded in a specific ratio.
Recognizing the Elements
The chemical formula, KMnO4, clearly indicates the presence of:
- One Potassium atom (K)
- One Manganese atom (Mn)
- Four Oxygen atoms (O)
Knowing this elemental composition is fundamental to determining the oxidation state of Manganese.
Assigning Known Oxidation Numbers
With the elements identified, we can now assign known oxidation numbers to Potassium and Oxygen. These are relatively constant in most compounds. This knowledge is critical for the next calculation steps.
Potassium (K): +1
Potassium is an alkali metal (Group 1) element. Alkali metals consistently exhibit a +1 oxidation state in compounds.
This stems from their tendency to lose one electron to achieve a stable electron configuration. Therefore, we can confidently assign Potassium an oxidation number of +1 in KMnO4.
Oxygen (O): -2
Oxygen is a highly electronegative element. It readily gains electrons to complete its octet. In most compounds, Oxygen exhibits an oxidation number of -2.
There are some exceptions (such as in peroxides, where it’s -1, or with Fluorine, where it’s positive). However, in KMnO4, Oxygen’s oxidation number is -2.
Having established the oxidation numbers for Potassium (+1) and Oxygen (-2), we are now prepared to calculate the oxidation number of Manganese (Mn). This will be the unknown variable in our equation.
Potassium and Oxygen, with their reliably consistent oxidation numbers, serve as anchors that allow us to determine the oxidation state of Manganese within the KMnO4 molecule. With these established values, we can now proceed to calculate the oxidation number of Mn.
Calculating Mn’s Oxidation Number: A Step-by-Step Guide
Our goal is to determine the oxidation number of Manganese (Mn) in Potassium Permanganate (KMnO4). Remember that the sum of the oxidation numbers of all atoms in a neutral compound must equal zero. We’ll use this principle, along with the known oxidation numbers of Potassium and Oxygen, to solve for Mn.
Setting Up the Equation
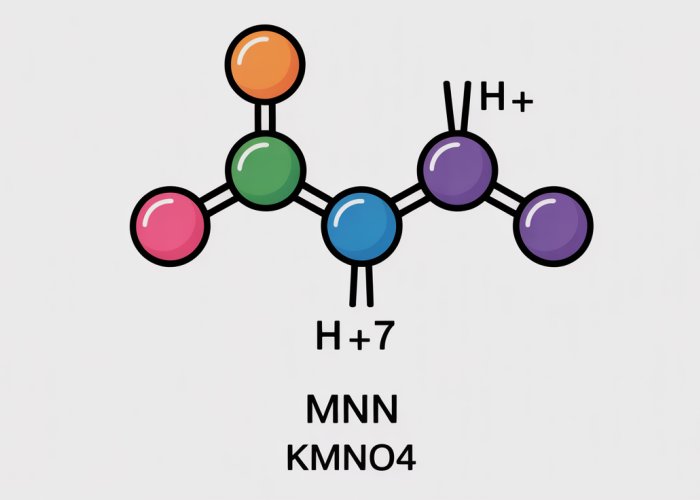
We begin by setting up an equation that represents the sum of the oxidation numbers in KMnO4. Let "Mn" represent the unknown oxidation number of Manganese. The equation will look like this:
(+1) + (Mn) + 4(-2) = 0
This equation represents the following:
- (+1): The oxidation number of Potassium (K).
- (Mn): The unknown oxidation number of Manganese (Mn).
- 4(-2): Four Oxygen atoms (O), each with an oxidation number of -2.
Solving for Mn: Isolating the Unknown
Now, let’s simplify and solve the equation for Mn:
1 + Mn – 8 = 0
Combine the constants:
Mn – 7 = 0
To isolate Mn, we add 7 to both sides of the equation:
Mn = +7
Therefore, the oxidation number of Manganese in KMnO4 is +7. This is a crucial result that dictates KMnO4’s chemical behavior.
A Detailed Step-by-Step Calculation Process
Let’s break down the calculation process into even smaller steps for absolute clarity:
- Identify the Knowns:
- Potassium (K) = +1
- Oxygen (O) = -2
- Write the Formula: KMnO4
- Set up the Equation: (+1) + (Mn) + 4(-2) = 0
- Simplify: 1 + Mn – 8 = 0
- Combine Constants: Mn – 7 = 0
- Isolate Mn: Mn = +7
- State the Result: The oxidation number of Mn in KMnO4 is +7.
By meticulously following these steps, you can confidently determine the oxidation number of Manganese in Potassium Permanganate. This skill is fundamental for understanding the compound’s reactivity and role in chemical reactions.
Our journey through the elemental composition and oxidation state calculations of Potassium Permanganate has revealed that the oxidation number of Manganese in KMnO4 is +7. This is a crucial result that dictates KMnO4’s chemical behavior.
Why Mn’s Oxidation Number Matters: The Power of Permanganate
The oxidation number of Manganese (Mn) in Potassium Permanganate (KMnO4) is not merely a numerical curiosity; it’s the key to understanding the compound’s remarkable chemical behavior. It directly connects to KMnO4’s potent ability to act as an oxidizing agent, a property that makes it valuable in various chemical reactions and applications.
Manganese as the Central Player
Manganese sits at the heart of the permanganate ion (MnO4-), a tetrahedral structure where the manganese atom is surrounded by four oxygen atoms. This central position is significant because the oxidation state of Mn dictates the overall charge distribution and reactivity of the entire ion.
The +7 oxidation state indicates that Manganese has a strong affinity for electrons. It is in its highest possible oxidation state in this compound, which makes it extremely reactive towards accepting electrons from other substances.
Oxidation Number and Oxidizing Power
A high positive oxidation number, like the +7 exhibited by Manganese in KMnO4, signifies a strong ability to attract and accept electrons. This is precisely what defines an oxidizing agent. KMnO4 readily accepts electrons from other substances, causing them to be oxidized (lose electrons) while itself being reduced (gaining electrons).
The greater the ability to accept electrons, the stronger the oxidizing agent. The oxidation number, therefore, provides a direct measure of KMnO4’s oxidizing power. In short, the +7 oxidation number of Mn is directly responsible for KMnO4’s effectiveness as an oxidizing agent.
Redox Reactions and Electron Acceptance
The term redox reaction refers to any chemical reaction that involves both reduction and oxidation. In a redox reaction, one substance loses electrons (oxidation), and another substance gains electrons (reduction). KMnO4 excels at facilitating these reactions because the Manganese atom readily accepts electrons from other reactants.
When KMnO4 acts as an oxidizing agent, the Manganese atom undergoes reduction. Its oxidation number decreases as it gains electrons. For instance, in acidic solutions, MnO4- is often reduced to Mn2+, where the oxidation number of Manganese changes from +7 to +2.
This acceptance of electrons from other reactants causes those reactants to undergo oxidation. The interplay between oxidation and reduction makes KMnO4 an invaluable tool in many chemical processes, from titrations in analytical chemistry to disinfection in water treatment.
Oxygen’s Influence: Electronegativity and Charge in Potassium Permanganate
Having established manganese as the central reactive site in KMnO4, it’s crucial to consider the supporting role of oxygen. Oxygen’s properties and oxidation state have a profound impact on KMnO4’s overall behavior.
The Electronegativity Powerhouse: Oxygen
Oxygen is one of the most electronegative elements on the periodic table. Electronegativity refers to an atom’s ability to attract electrons within a chemical bond.
Oxygen’s high electronegativity compels it to strongly pull electrons towards itself when bonding with other elements, including manganese and potassium in KMnO4. This electron-attracting behavior is fundamental to understanding oxygen’s consistent oxidation state.
The Consistent -2 Oxidation State of Oxygen
In most compounds, oxygen exhibits a -2 oxidation state. This means that each oxygen atom effectively gains two electrons in its interactions with other elements.
This consistent -2 charge is a direct consequence of its electron configuration and its drive to achieve a stable octet. The consistent oxidation state simplifies calculations and allows accurate charge distribution predictions.
Oxygen’s Contribution to KMnO4’s Overall Charge and Reactivity
The four oxygen atoms in the permanganate ion (MnO4-) collectively contribute a -8 charge (4 x -2 = -8). This significant negative charge directly influences the electronic environment around the central manganese atom.
To maintain electrical neutrality within the KMnO4 compound, the potassium ion (K+) provides a +1 charge. Thus, the manganese atom is forced into a +7 oxidation state to balance the overall charge: (+1) + (+7) + (-8) = 0.
The substantial positive charge on manganese, driven by oxygen’s electronegativity, makes it a potent electron acceptor, which is the hallmark of an oxidizing agent.
Oxygen’s Role in Redox Reactions: Facilitating Electron Transfer
In redox reactions involving KMnO4, oxygen atoms play a critical, albeit indirect, role. They do not typically undergo changes in their oxidation state themselves.
Instead, they maintain the highly electronegative environment that forces manganese to accept electrons from other reacting species. This electron acceptance by manganese is what drives the oxidation of other substances, thus allowing oxygen to drive KMnO4’s role as the oxidizing agent.
Essentially, oxygen’s consistent pull on electrons from manganese makes it easier for manganese to grab electrons from other substances. Oxygen’s electronegativity facilitates the electron transfer that is characteristic of redox reactions.
KMnO4 in Action: Unveiling Redox Reactions
The interplay of potassium permanganate (KMnO4) with other substances showcases its remarkable ability to facilitate redox reactions. In essence, KMnO4 acts as a powerful oxidizing agent, readily accepting electrons from other reactants.
This electron-grabbing capability is directly linked to manganese’s oxidation state and its eagerness to transition to a more stable form. Let’s delve into specific examples to illustrate this phenomenon.
KMnO4 as a Powerful Oxidizing Agent: Electron Acceptance
As an oxidizing agent, KMnO4 causes the oxidation of another substance. This means the other substance loses electrons. Simultaneously, KMnO4 itself is reduced, gaining those electrons.
This electron transfer is the heart of a redox reaction. The permanganate ion (MnO4-) is particularly effective at this because manganese (Mn) can exist in multiple oxidation states.
Redox Reaction Examples: A Showcase of KMnO4’s Power
To truly grasp KMnO4’s role, let’s examine a few common redox reactions:
Oxidation of Iron(II) to Iron(III)
One classic example involves the oxidation of iron(II) ions (Fe2+) to iron(III) ions (Fe3+) in an acidic solution. KMnO4 readily oxidizes Fe2+, converting it to Fe3+.
The balanced equation for this reaction is:
5 Fe2+(aq) + MnO4-(aq) + 8 H+(aq) → 5 Fe3+(aq) + Mn2+(aq) + 4 H2O(l)
Oxidation of Oxalate Ions
Another important reaction is the oxidation of oxalate ions (C2O42-) to carbon dioxide (CO2) in an acidic environment. Permanganate effectively breaks down oxalate.
The balanced equation is:
2 MnO4-(aq) + 5 C2O42-(aq) + 16 H+(aq) → 2 Mn2+(aq) + 10 CO2(g) + 8 H2O(l)
Decolorization of Alkenes
KMnO4 can also oxidize alkenes (compounds containing carbon-carbon double bonds). This reaction results in the decolorization of the purple permanganate solution. This is a common qualitative test for the presence of unsaturation in organic molecules.
The Changing Oxidation Number of Manganese
Observe how the oxidation number of manganese changes during these reactions. In the permanganate ion (MnO4-), manganese has an oxidation state of +7.
After accepting electrons, manganese is typically reduced to Mn2+ ions in acidic solution, where its oxidation state becomes +2. This represents a significant decrease in oxidation number, confirming KMnO4’s role as an oxidizing agent. The change in oxidation number, from +7 to +2, shows that each manganese atom gains 5 electrons during the redox process.
Balancing Redox Reactions Involving KMnO4
Balancing redox reactions, particularly those involving KMnO4, can be challenging. Two primary methods are used: the half-reaction method and the oxidation number method.
The half-reaction method breaks the overall reaction into two separate half-reactions: one for oxidation and one for reduction. Each half-reaction is balanced separately, and then the two are combined to yield the balanced overall equation.
The oxidation number method relies on tracking the changes in oxidation numbers of the elements involved. By equating the total increase in oxidation number with the total decrease, stoichiometric coefficients can be determined. Balancing redox reactions is essential to accurately represent the chemical changes occurring.
Avoiding Pitfalls: Common Mistakes in Oxidation Number Calculations
Having explored the power of KMnO4 and the role of manganese’s oxidation state, it’s crucial to address common errors that can arise when calculating oxidation numbers. These mistakes can lead to misunderstandings of chemical behavior and inaccurate predictions of reactivity. Let’s examine frequent pitfalls and provide strategies for accurate calculations, especially within the context of KMnO4.
The Perils of Overlooking Overall Charge
One of the most frequent errors stems from neglecting the overall charge of the ion or molecule. The sum of the oxidation numbers of all atoms in a neutral molecule must equal zero. For an ion, the sum must equal the ion’s charge.
For example, in KMnO4, the molecule is neutral, so the oxidation numbers must sum to zero. However, if you were dealing with the permanganate ion, MnO4-, the oxidation numbers must sum to -1. Failing to account for this overall charge will inevitably lead to incorrect results.
Misapplication of Oxidation State Rules
While the rules for assigning oxidation numbers are generally straightforward, misapplication can occur. One common mistake is assuming oxygen always has an oxidation state of -2.
While this is overwhelmingly true, there are exceptions, such as in peroxides (like H2O2), where oxygen has an oxidation state of -1. It’s critical to know when to apply exceptions to the general rules.
Another frequent error involves elements with variable oxidation states. Manganese is a prime example; it can exist in multiple oxidation states. Do not assume a specific oxidation number without calculation.
The Importance of Proper Algebra
Calculating oxidation numbers involves basic algebra. Errors in arithmetic can be surprisingly common. Double-check your work, particularly when dealing with multiple oxygen atoms or complex ions.
For example, in KMnO4, the calculation is: (+1) + Mn + 4(-2) = 0. Simple algebraic errors can easily lead to an incorrect oxidation number for Mn.
Tips and Tricks for Accurate Calculations
To avoid these pitfalls, consider these tips and tricks:
-
Always start with known oxidation numbers: Begin by assigning oxidation numbers to elements with well-defined oxidation states, such as alkali metals (+1) and oxygen (-2 in most cases).
-
Write out the equation clearly: Explicitly write out the algebraic equation representing the sum of oxidation numbers. This helps visualize the calculation and minimize errors.
-
Double-check your arithmetic: Carefully review your calculations, paying attention to signs and multiplication.
-
Consider the context: Be mindful of the chemical environment. Are you dealing with a neutral molecule, an ion, or a complex compound? This influences the overall charge you must account for.
-
Practice, practice, practice: The more you practice calculating oxidation numbers, the more comfortable and accurate you will become.
A KMnO4 Calculation Checklist
To specifically ensure accurate KMnO4 calculations, utilize this checklist:
- Confirm the overall charge of the species (0 for KMnO4).
- Assign +1 to Potassium (K).
- Assign -2 to Oxygen (O).
- Set up the equation: (+1) + (Mn) + 4(-2) = 0
- Solve for Mn.
- Double-check the algebra.
- Confirm that the sum of all oxidation numbers equals the overall charge.
By understanding these common mistakes and implementing the suggested tips, you can confidently and accurately determine oxidation numbers, leading to a deeper comprehension of chemical reactions and the role of compounds like KMnO4.
FAQs About KMnO4 Oxidation Number
Still have questions about understanding the oxidation number of KMnO4? Here are some frequently asked questions to help clarify the concept.
Why is it important to know the oxidation number of Mn in KMnO4?
Knowing the oxidation number of Mn in KMnO4 is crucial because it helps you predict and understand how KMnO4 will behave in chemical reactions, particularly redox reactions. KMnO4 is a strong oxidizing agent, and its oxidizing power directly relates to the Mn oxidation number.
What does the oxidation number of potassium (K) contribute to determining the kmno4 oxidation number of Mn?
Potassium (K) always has an oxidation number of +1. This known value, along with the known oxidation number of oxygen (-2), allows us to set up an equation and solve for the unknown oxidation number of manganese (Mn) in KMnO4.
If the oxidation number of oxygen is usually -2, are there exceptions that change the kmno4 oxidation number calculation?
While oxygen usually has an oxidation number of -2, there are rare exceptions like in peroxides (where it’s -1) or with fluorine (where it’s positive). However, in KMnO4, oxygen’s oxidation number is -2, ensuring the manganese oxidation number is +7.
Can the oxidation number of Mn in KMnO4 ever be different than +7?
In KMnO4 itself, the oxidation number of Mn will always be +7. However, in other manganese-containing compounds, its oxidation number can vary. The +7 oxidation state is specific to the permanganate ion (MnO4-).
So, there you have it! Hopefully, this guide helped clear up any confusion about the kmno4 oxidation number. Now you can tackle those redox reactions with confidence!