The electronegativity of Fluorine, a key property studied extensively by the Linus Pauling scale, directly influences the formation of the fluorine ion charge. This negative charge, characterized within the framework of quantum mechanics, dictates its interactions. In particular, the resulting negative charge of the fluorine ion charge, which is analyzed using computational chemistry software such as Gaussian, has a profound effect on the formation of the fluoride compound and a significant amount of chemical reaction properties, influencing processes throughout various fields.
Understanding the Fluorine Ion Charge: Impact and Implications
Fluorine, a highly reactive element, readily forms ions. The resulting "fluorine ion charge" significantly impacts its chemical behavior and interactions with other substances. This exploration delves into the specifics of this charge, its origins, and its surprising consequences.
The Formation of the Fluoride Ion (F-)
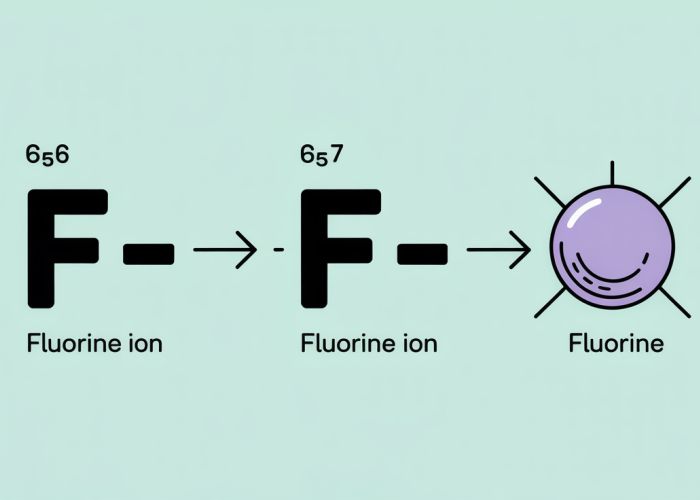
The "fluorine ion charge" is directly linked to the process of fluorine gaining an electron to achieve a stable electron configuration.
- Atomic Structure: Fluorine (F) has an atomic number of 9, meaning it possesses 9 protons and 9 electrons in its neutral state. Its electron configuration is 1s²2s²2p⁵.
- The Octet Rule: Atoms tend to gain, lose, or share electrons to achieve a full outer electron shell (octet rule), resembling the stable noble gas configuration.
- Electron Gain: Fluorine needs just one more electron to complete its 2p subshell and achieve the same electron configuration as Neon (Ne), a noble gas.
- Charge Development: When fluorine gains one electron, it now has 10 electrons and 9 protons. This imbalance results in a net negative charge of -1. This negatively charged ion is called a fluoride ion (F⁻).
Characteristics of the -1 Charge
The -1 "fluorine ion charge" isn’t arbitrary. It reflects a fundamental energetic preference.
- Electronegativity: Fluorine is the most electronegative element. This means it has the highest tendency to attract electrons towards itself in a chemical bond. Its electronegativity value (Pauling scale) is around 3.98.
- Ionization Energy vs. Electron Affinity: Ionization energy is the energy required to remove an electron from an atom. Electron affinity is the energy change when an electron is added to an atom. Fluorine has a high electron affinity and relatively low ionization energy (although higher than other halogens). This combination energetically favors the formation of the F⁻ ion.
- Electrostatic Forces: The negative charge creates a strong electrostatic attraction between the fluoride ion and positively charged species (cations).
Impact of the Fluorine Ion Charge
The -1 "fluorine ion charge" influences a variety of chemical and biological processes.
Chemical Reactivity
- Ionic Bond Formation: Fluoride readily forms ionic bonds with metals, creating salts like sodium fluoride (NaF) and calcium fluoride (CaF₂). The strong electrostatic attraction between F⁻ and the metal cation results in stable, high-melting-point compounds.
- Hydrogen Bonding: Fluoride can participate in hydrogen bonding, though its ability to do so is influenced by its environment (e.g., whether it is hydrated). While weaker than ionic bonds, hydrogen bonds involving fluoride are important in biological systems.
Biological Effects
- Tooth Enamel Hardening: Fluoride ions are added to toothpaste and drinking water to strengthen tooth enamel. Hydroxyapatite, the main component of enamel, can incorporate fluoride to form fluorapatite. Fluorapatite is more resistant to acid attack from bacteria, reducing the risk of tooth decay. This is a direct consequence of the "fluorine ion charge" enabling strong interactions with calcium ions in the enamel.
- Enzyme Inhibition: In some biological systems, fluoride ions can inhibit enzymes. This occurs because fluoride can bind to active sites or otherwise alter the enzyme’s structure, often by complexing with metal ions required for enzyme function. The "fluorine ion charge" facilitates this binding.
- Toxicity: High concentrations of fluoride can be toxic. Excessive fluoride intake can lead to skeletal fluorosis and other health problems. This is partly due to fluoride’s ability to interfere with calcium metabolism and other essential biological processes.
Environmental Considerations
- Water Contamination: Naturally occurring fluoride can be found in groundwater. In some regions, high fluoride concentrations can pose a health risk to populations consuming untreated water. Industrial processes, such as aluminum production, can also release fluoride into the environment.
- Remediation Strategies: Various methods are employed to remove fluoride from contaminated water, including adsorption, precipitation, and membrane filtration. These methods often target the "fluorine ion charge" using materials with a high affinity for fluoride ions.
| Property | Description | Relevance to Fluorine Ion Charge |
|---|---|---|
| Electronegativity | The ability of an atom to attract electrons towards itself in a chemical bond. | High electronegativity drives F⁻ formation. |
| Ionic Radius | The radius of an ion in a crystal lattice. | Affects interactions with other ions. |
| Hydrogen Bonding | Attraction between hydrogen bound to an electronegative atom and another electronegative atom. | Influences biological activity. |
| Toxicity | The degree to which a substance can damage an organism. | Related to F⁻ interference with biological processes. |
Fluorine Ion Charge: Frequently Asked Questions
Here are some common questions about the impact of the fluorine ion charge and its role in various processes.
What is the charge of a fluorine ion, and why is it important?
A fluorine ion has a charge of -1 (written as F⁻). This negative charge is crucial because it makes fluorine highly reactive, allowing it to readily form ionic bonds with positively charged ions. The fluorine ion charge is fundamental to its reactivity.
How does the fluorine ion charge affect its ability to form compounds?
Due to its -1 charge, a fluorine ion readily attracts positively charged ions. This strong attraction allows fluorine to form stable ionic compounds like sodium fluoride (NaF), where the positive sodium ion balances the negative fluorine ion charge.
Why is the reactivity of fluorine, related to its ion charge, considered "surprising"?
Fluorine’s extreme reactivity, stemming from its fluorine ion charge, can be surprising because it’s far more reactive than other halogens like chlorine. This high reactivity allows it to react even with noble gases under specific conditions, forming unique compounds.
In what applications is understanding the fluorine ion charge particularly important?
Understanding the fluorine ion charge is essential in diverse fields. It is important in the design of new materials with specific properties, like Teflon (a fluoropolymer). Also, in drug development, manipulating the fluorine ion charge can modify a drug’s effectiveness.
Hopefully, this sheds some light on the often-overlooked importance of the fluorine ion charge! Now you’ve got a better understanding of why that negative charge matters so much. Go forth and apply that knowledge!