Chemical reactions represent the foundation of countless processes, ranging from the rusting of iron to the energy production in cellular respiration. These reactions, at their core, involve the interaction of reactants, which can be categorized by their enthalpy change. This categorization gives rise to the concepts of exothermic vs endothermic reactions, where one releases energy and the other absorbs it from the surroundings. Understanding exothermic vs endothermic processes is crucial for applications across diverse fields like chemical engineering and sustainable energy development.
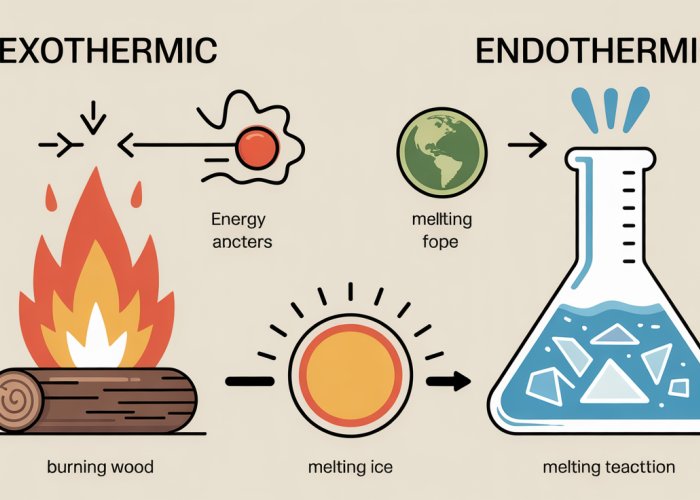
Consider the comforting warmth of a crackling fireplace on a chilly evening. The burning wood, a classic example of an exothermic reaction, releases energy in the form of heat and light, creating a cozy atmosphere.
Now, picture a refreshing glass of iced tea on a sweltering summer day. The melting ice cubes, an endothermic reaction, absorb heat from the surrounding tea, providing a cooling effect.
These everyday experiences offer a glimpse into the fascinating world of chemical reactions and the fundamental differences between exothermic and endothermic processes.
Defining Exothermic and Endothermic Reactions
At their core, chemical reactions involve the breaking and forming of chemical bonds, accompanied by changes in energy.
Exothermic reactions are those that release energy, typically in the form of heat, into the surroundings. Think of them as energy producers, increasing the temperature of their environment.
In contrast, endothermic reactions absorb energy from their surroundings, often leading to a decrease in temperature. They act as energy consumers, requiring an input of energy to proceed.
The Heart of the Matter: Energy Flow and Chemical Change
The distinction between exothermic and endothermic reactions lies in the direction of energy flow.
Exothermic reactions release energy as the system moves to a lower energy state.
Endothermic reactions require energy to reach a higher energy state.
This article will explore the fundamental differences between exothermic and endothermic reactions, examining their energy flow, impact on temperature, and significance in various chemical processes. We will delve into the underlying principles that govern these reactions and explore their far-reaching implications in chemistry and beyond.
That comforting fireplace and the cooling iced tea serve as tangible reminders that chemical reactions aren’t just theoretical concepts, they are happening all around us, constantly shaping our world. Understanding the energy dynamics of these reactions, particularly the release or absorption of heat, is crucial to grasping the fundamental principles of chemistry. Let’s begin by exploring the world of exothermic reactions.
Exothermic Reactions: Heat’s Outward Journey
Exothermic reactions are chemical processes that release energy into their surroundings, most commonly in the form of heat. This release of energy is a key characteristic, distinguishing them from their endothermic counterparts.
Defining Exothermic Reactions
At its core, an exothermic reaction is a chemical transformation where the total energy of the products is less than the total energy of the reactants. This energy difference is released into the surroundings, often manifesting as heat, light, or sound.
Think of it like this: the reactants are initially at a higher energy level, and as they transform into products, they "fall" to a lower energy level, releasing the excess energy along the way.
The Release of Heat
The defining feature of exothermic reactions is the liberation of heat into the surrounding environment. This occurs as the chemical bonds in the reactants are broken and new, more stable bonds are formed in the products. The formation of these new bonds releases more energy than was required to break the initial bonds.
Impact on Surrounding Temperature
The release of heat in an exothermic reaction directly impacts the temperature of the immediate surroundings. As energy is transferred from the reacting system to the environment, the temperature increases. This temperature increase is a readily observable indication that an exothermic reaction is taking place.
The extent of the temperature increase depends on several factors, including the amount of reactants involved, the specific reaction taking place, and the thermal properties of the surroundings.
Examples of Common Exothermic Reactions
Exothermic reactions are prevalent in our daily lives and various industrial processes. Here are a few prominent examples:
Combustion
Combustion, or burning, is a classic example of an exothermic reaction. When fuels like wood, propane, or natural gas react with oxygen, they release a significant amount of heat and light.
This process is used to generate power in engines and power plants, as well as to provide warmth in homes.
Explosions
Explosions are rapid exothermic reactions that produce a large volume of gas in a short period, generating a powerful expansion. Examples include the detonation of dynamite or the ignition of fireworks.
The sudden release of energy creates a shockwave and significant heat, resulting in the characteristic explosive effect.
Neutralization Reactions
Neutralization reactions, such as the reaction between an acid and a base, are also exothermic. When an acid and a base combine, they produce salt and water, releasing heat in the process.
This principle is utilized in various chemical processes and is essential for maintaining pH balance in different systems.
That comforting fireplace and the cooling iced tea serve as tangible reminders that chemical reactions aren’t just theoretical concepts, they are happening all around us, constantly shaping our world. Understanding the energy dynamics of these reactions, particularly the release or absorption of heat, is crucial to grasping the fundamental principles of chemistry. Let’s begin by exploring the world of exothermic reactions.
Endothermic Reactions: Heat’s Inward Absorption
While exothermic reactions release energy like a burst of warmth, endothermic reactions operate in reverse. They represent a class of chemical processes that absorb energy from their surroundings, leading to a noticeable cooling effect.
Defining Endothermic Reactions
At its most fundamental level, an endothermic reaction is a chemical transformation where the total energy of the products is greater than the total energy of the reactants. This energy difference is not created from nothing; it’s drawn in from the surrounding environment.
Think of it as the reactants needing an energy "boost" to climb to a higher energy level and transform into the products. This required energy is extracted from the immediate environment.
The Absorption of Heat
The defining characteristic of an endothermic reaction is its absorption of heat from the surrounding environment. This occurs as the chemical bonds in the reactants are broken, requiring an input of energy to facilitate the change.
The energy used to break these bonds is greater than the energy released when new, less stable bonds are formed in the products. This imbalance results in a net absorption of energy as heat from the surroundings.
Impact on Surrounding Temperature
The absorption of heat in an endothermic reaction directly impacts the temperature of its immediate environment.
As the reaction progresses, it draws energy from the surroundings, causing the temperature to drop. This cooling effect is often readily noticeable and can be measured using thermometers or other temperature-sensitive devices.
In essence, the surroundings act as a heat source, providing the necessary energy for the endothermic reaction to proceed.
Examples of Endothermic Reactions
Endothermic reactions are prevalent in both natural and artificial systems. Some notable examples include:
-
Photosynthesis: The process by which plants convert carbon dioxide and water into glucose and oxygen. This reaction requires energy from sunlight to occur.
-
Melting Ice: The phase transition of solid ice to liquid water requires heat to break the hydrogen bonds holding the water molecules in a crystalline structure.
-
Evaporation of Water: Similar to melting, the evaporation of liquid water into gaseous water vapor requires energy to overcome the intermolecular forces between water molecules.
-
Cooking (Baking a Cake): The chemical changes that occur during baking, such as the breakdown of proteins and starches, require heat from the oven to proceed.
That leaves us with a clear understanding of how energy flows in and out of a chemical system, influencing the temperature we observe. But how do we quantify this energy change? What is the yardstick by which we measure the heat absorbed or released during these transformations? The answer lies in the concept of enthalpy.
Enthalpy: The Heat Within
Enthalpy, often symbolized as H, is a thermodynamic property of a system that represents the total heat content of the system at constant pressure. It’s a comprehensive measure that includes the internal energy of the system (the energy associated with the motion and interactions of its molecules) as well as the energy associated with pressure and volume.
Defining Enthalpy
Mathematically, enthalpy is defined as:
H = U + PV
Where:
- H represents enthalpy.
- U represents the internal energy of the system.
- P represents the pressure of the system.
- V represents the volume of the system.
While we might not always know the absolute value of enthalpy, we are primarily interested in the change in enthalpy (ΔH) during a chemical reaction. This change tells us whether heat is absorbed or released.
The Role of Enthalpy in Chemical Reactions
In chemical reactions, enthalpy plays a crucial role in determining the heat flow between the system and its surroundings. The change in enthalpy (ΔH) represents the amount of heat absorbed or released during a reaction carried out at constant pressure.
This change in enthalpy is directly related to whether a reaction is exothermic or endothermic.
- A negative ΔH indicates an exothermic reaction (heat is released).
- A positive ΔH indicates an endothermic reaction (heat is absorbed).
Enthalpy as a State Function
One of the most important aspects of enthalpy is that it is a state function. This means that the change in enthalpy (ΔH) depends only on the initial and final states of the system, and not on the path taken to get from one state to the other.
Imagine climbing a mountain: the change in your elevation (your "enthalpy") is the same whether you take a direct route straight up or a winding path around.
This property makes enthalpy incredibly useful for calculating heat changes in complex reactions, as we only need to know the starting and ending points. We can calculate ΔH by subtracting the enthalpy of the reactants from the enthalpy of the products:
ΔH = H(products) – H(reactants)
This simple equation provides a powerful tool for understanding and predicting the heat flow in chemical reactions.
That change in enthalpy is directly related to whether a reaction is exothermic or endothermic. Understanding enthalpy change allows us to predict and quantify the heat released or absorbed in a chemical process, providing valuable insights into the energy dynamics of the reaction. With that in mind, let’s explore how enthalpy changes specifically in exothermic reactions.
Exothermic Reactions and Enthalpy Change (ΔH)
In exothermic reactions, the system releases heat to its surroundings. This release of energy has a direct and predictable impact on the enthalpy of the system.
Enthalpy Decrease in Exothermic Reactions
The key characteristic of an exothermic reaction is that the enthalpy of the system decreases during the reaction. The system is losing heat, which means that the total heat content of the system is lower after the reaction than before.
This decrease in enthalpy is a direct consequence of the conversion of chemical energy into thermal energy, which is then released as heat.
The Negative Sign of ΔH
To express this decrease in enthalpy mathematically, we use the symbol ΔH (delta H), which represents the change in enthalpy.
For exothermic reactions, ΔH is always negative (ΔH < 0).
This negative sign is a convention that signifies that heat is being released from the system. The magnitude of ΔH indicates the amount of heat released per mole of reaction.
Energy Levels of Reactants and Products
The negative ΔH value also tells us something important about the relative energy levels of the reactants and products.
In an exothermic reaction, the products have lower energy than the reactants.
This difference in energy is what drives the release of heat. The system is moving from a higher energy state (the reactants) to a lower energy state (the products), and the excess energy is released as heat.
Think of it like a ball rolling downhill: it starts at a higher potential energy and moves to a lower potential energy, releasing energy in the process.
Similarly, in exothermic reactions, the system moves to a lower energy state, releasing energy as heat.
The impact of this heat release on enthalpy is predictable; therefore, understanding what happens with exothermic reactions and enthalpy is key to then understanding endothermic reactions. Let’s now turn our attention to the energetic changes in endothermic reactions and how they relate to enthalpy.
Endothermic Reactions and Enthalpy Change (ΔH)
In contrast to exothermic reactions, endothermic reactions absorb heat from their surroundings.
This absorption of energy has a distinct effect on the enthalpy of the system.
Enthalpy Increase in Endothermic Reactions
The defining characteristic of an endothermic reaction is that the enthalpy of the system increases during the reaction.
The system gains heat, meaning that the total heat content of the system is higher after the reaction than it was before.
This increase in enthalpy is a direct result of the conversion of thermal energy into chemical energy, which is then stored within the products.
The Positive Sign of ΔH
To represent this increase in enthalpy mathematically, we again use the symbol ΔH (delta H), representing the change in enthalpy.
However, for endothermic reactions, ΔH is always positive (ΔH > 0).
This positive sign is the convention to indicate that heat is being absorbed into the system.
The magnitude of ΔH indicates the amount of heat absorbed per mole of reaction.
Energy Levels of Reactants and Products
The positive ΔH value also reveals a crucial insight into the relative energy levels of the reactants and products.
In an endothermic reaction, the products have a higher energy level than the reactants.
This means that energy has been added to the system to transform the reactants into the higher-energy products.
The absorbed energy is stored as chemical potential energy within the bonds of the products.
This higher energy state explains why endothermic reactions often require a continuous input of energy to proceed.
The Guiding Hand of Thermodynamics
While enthalpy provides a snapshot of energy change during a reaction, the underlying principles that govern whether a reaction will even occur, and the direction it will proceed, are rooted in the laws of thermodynamics. These laws act as the fundamental rules of the game, dictating the flow of energy and the feasibility of chemical transformations, including both exothermic and endothermic processes.
The First Law: Conservation of Energy
The first law of thermodynamics, also known as the law of conservation of energy, is paramount to understanding energy changes in chemical reactions. It states that energy cannot be created or destroyed; it can only be converted from one form to another.
In the context of chemical reactions, this means that the total energy of the universe (system + surroundings) remains constant throughout the reaction.
For an exothermic reaction, the energy released as heat doesn’t simply vanish; it is transferred to the surroundings, raising their temperature. Conversely, in an endothermic reaction, the energy absorbed from the surroundings doesn’t appear from nowhere; it is converted into chemical potential energy stored within the bonds of the products.
This principle highlights a crucial connection: Energy released by an exothermic reaction must be equal to the change in enthalpy of the system, but with an opposite sign. Similarly, the energy absorbed by an endothermic reaction reflects the increase in enthalpy within the system, drawn from the surroundings.
Implications for Chemical Reactions
The conservation of energy has several critical implications:
-
Energy Accounting: It provides a framework for tracking energy flow in chemical reactions, ensuring that all energy inputs and outputs are accounted for.
-
Predicting Feasibility: While the first law doesn’t dictate whether a reaction will occur, it sets the stage by establishing that any energy changes must adhere to its strict accounting principles. If a reaction violates the first law, it is simply impossible.
-
Quantifying Heat Transfer: The first law allows us to quantify the amount of heat transferred during a reaction, by equating it with the change in internal energy of the system and the work done by or on the system.
The first law sets the energetic stage for any reaction, dictating that the books must always balance: energy in equals energy out, even as it transforms between heat, chemical potential, and other forms. This law is a cornerstone of understanding the behavior of both exothermic and endothermic reactions.
The conservation of energy underscores the framework within which reactions operate, but it doesn’t tell the whole story. Just because a reaction can happen doesn’t mean it will. There’s often an initial hurdle, a spark needed to ignite the transformation.
Activation Energy: The Spark of Reaction
Chemical reactions, regardless of whether they release or absorb energy overall, generally don’t occur spontaneously the moment reactants are mixed. Consider lighting a match: the wood and oxygen are present, and the combustion reaction is exothermic, yet the match remains unlit until struck. The underlying principle governing this initial resistance is activation energy.
Defining Activation Energy
Activation energy is defined as the minimum amount of energy required to initiate a chemical reaction. It represents the energy "barrier" that must be overcome for the reaction to proceed from reactants to products.
Think of it as pushing a rock over a hill. The rock represents the reactants, and the other side of the hill, the products. Even if the other side is at a lower elevation (representing a release of energy), you still need to expend energy to push the rock up and over the crest.
The Transition State
The activation energy provides reactants with the kinetic energy needed to reach a transition state. The transition state is a high-energy, unstable intermediate state between reactants and products.
At the transition state, bonds in the reactants are partially broken, and new bonds are partially formed. It is a fleeting moment of molecular rearrangement, poised on the precipice of product formation.
Importance of Activation Energy
Activation energy is critical because it determines the rate at which a reaction will occur. A high activation energy means that only a small fraction of molecules will possess sufficient energy to react at a given temperature, resulting in a slow reaction.
Conversely, a low activation energy implies that many molecules will have enough energy to react, leading to a fast reaction. The activation energy therefore acts as a regulator, controlling the speed of chemical transformations.
Factors Influencing Activation Energy
Several factors can influence the activation energy of a reaction. These include:
- The nature of the reactants: Some molecules are inherently more stable and require more energy to break their bonds.
- Temperature: Higher temperatures provide molecules with more kinetic energy, increasing the likelihood of overcoming the activation energy barrier.
- Catalysts: Catalysts are substances that lower the activation energy of a reaction without being consumed themselves. They provide an alternative reaction pathway with a lower energy barrier, thereby speeding up the reaction.
In essence, activation energy dictates whether a collision between reactant molecules will be a successful one, leading to product formation, or simply a futile bounce. It’s the key to unlocking the potential energy stored within chemical bonds and driving the chemical processes that shape our world.
The activation energy provides reactants with the kinetic energy needed to reach a transition state. The transition state is a high-energy, unstable intermediate state between reactants and products.
At the transition state, bonds in the reactants are partially broken, and new bonds are partially formed. It is a fleeting moment of molecular rearrangement, poised on the precipice…
Activation Energy in Exothermic Reactions: The Jump Start
Exothermic reactions, characterized by their release of energy, might seem like they’d proceed effortlessly once the reactants are combined. However, the reality is often quite different. They, too, require an initial input of energy to get started.
This initial energy, the activation energy, plays a crucial role in determining the kinetics of these reactions.
Overcoming the Initial Hurdle
The activation energy in exothermic reactions can be visualized as a ‘hill’ that the reactants must climb before they can ‘fall’ into the lower energy state of the products.
While the overall energy change (ΔH) is negative, signifying a release of energy, the reaction cannot proceed spontaneously without first overcoming this energy barrier.
Consider the burning of wood, an oft-cited example. Simply placing wood in the presence of oxygen at room temperature will not result in combustion.
A match or some other form of ignition is necessary to provide the initial energy needed to break the existing bonds in the wood and oxygen molecules.
The Self-Sustaining Nature
What distinguishes exothermic reactions is that once the activation energy is supplied and the reaction begins, the heat released often provides enough energy to activate more reactant molecules.
This creates a chain reaction effect, allowing the reaction to become self-sustaining.
The initial heat released acts as a catalyst, lowering the activation energy for subsequent reactions.
This is why, for example, once a fire is started, it continues to burn until the fuel (wood) or the oxidizer (oxygen) is exhausted.
Activation Energy and Reaction Rate
The magnitude of the activation energy has a direct impact on the reaction rate.
A lower activation energy means that more molecules possess sufficient energy to overcome the barrier at a given temperature, leading to a faster reaction.
Conversely, a higher activation energy implies that fewer molecules have enough energy, resulting in a slower reaction.
Therefore, while exothermic reactions release energy, the rate at which they proceed is dictated by the activation energy required to initiate the process.
Activation Energy in Endothermic Reactions: Continuous Input Required
We’ve explored how exothermic reactions, once jump-started by activation energy, often become self-sustaining due to the heat they release. But what about endothermic reactions? Do they behave similarly after surpassing their activation energy barrier?
The answer reveals a critical distinction between the two types of reactions.
Sustaining the Reaction: A Constant Energy Supply
Unlike their exothermic counterparts, endothermic reactions typically demand a continuous supply of energy to proceed at a noticeable rate, even after the activation energy has been provided.
Think of it like pushing a boulder uphill. The initial push (activation energy) gets it moving.
But if you stop pushing, the boulder will roll back down. Endothermic reactions are similar.
Without constant energy input, they will either slow dramatically or cease altogether.
Why the Need for Constant Input?
This requirement for continuous energy stems directly from the nature of endothermic reactions. They absorb heat from their surroundings.
As the reaction progresses, it depletes the thermal energy of its environment.
This cooling effect, if not counteracted, hinders the reaction’s ability to continue breaking bonds and forming new ones.
The reaction essentially starves itself of the energy it needs to proceed.
Examples in Action: Demonstrating Continuous Input
Several common examples illustrate this principle.
Consider the melting of ice. While initial heat (activation energy) is needed to begin the phase transition from solid to liquid, the process will only continue if heat is constantly supplied.
Remove the heat source, and the water will refreeze.
Similarly, in the process of cooking, baking a cake requires a constant input of thermal energy from the oven.
If the oven is turned off prematurely, the cake will not finish baking properly. The endothermic reactions involved in leavening and setting the cake’s structure simply won’t complete.
Implications for Reaction Conditions
The need for continuous energy input has significant implications for how endothermic reactions are conducted in both laboratory and industrial settings.
Maintaining a consistent temperature or energy source is often crucial for achieving optimal reaction yields and rates.
This might involve using specialized heating equipment or carefully controlling the reaction environment to ensure a steady flow of energy into the system.
Practical Applications and Considerations
Understanding this characteristic of endothermic reactions is not merely an academic exercise.
It is essential for designing and optimizing various processes, from chemical synthesis to industrial manufacturing.
For instance, in the production of certain polymers or pharmaceuticals, carefully managing the energy input can be the key to achieving desired product quality and efficiency.
In essence, while activation energy provides the initial spark, consistent energy supply is the fuel that keeps endothermic reactions running. Recognizing and addressing this distinction is fundamental to harnessing the power of these reactions effectively.
The examples given within should be as varied as possible.
Bond Energy: The Glue That Holds It Together
Having examined the energetic demands of sustaining endothermic reactions, it becomes apparent that understanding the energy stored within chemical bonds themselves is crucial. These bonds, acting as the very glue holding molecules together, dictate the energy landscape of all chemical reactions.
Defining Bond Energy
At its core, bond energy is defined as the amount of energy needed to break one mole of a specific type of bond in a gaseous molecule.
It’s an endothermic process, meaning energy must be supplied to overcome the attractive forces holding the atoms together.
This energy is typically expressed in units of kilojoules per mole (kJ/mol).
The Strength of Attraction
Bond energy provides a quantitative measure of the strength of a chemical bond.
A higher bond energy indicates a stronger bond, requiring more energy to break.
Conversely, a lower bond energy suggests a weaker bond, which is more easily broken.
Single, Double, and Triple Bonds
The type of chemical bond dramatically impacts its energy. Single bonds generally have lower bond energies than double bonds, which, in turn, have lower bond energies than triple bonds.
This is because multiple bonds involve a greater number of shared electrons, resulting in a stronger attractive force between the atoms.
For example, consider the carbon-carbon bonds:
- A C-C single bond has a bond energy of approximately 347 kJ/mol.
- A C=C double bond has a bond energy of approximately 614 kJ/mol.
- A C≡C triple bond has a bond energy of approximately 839 kJ/mol.
Factors Influencing Bond Energy
Several factors can influence the strength of a chemical bond and, therefore, its bond energy.
These include:
Electronegativity
The difference in electronegativity between the bonded atoms plays a significant role. Larger electronegativity differences often lead to more polar bonds, which can be stronger and have higher bond energies.
Atomic Size
Smaller atoms tend to form stronger bonds due to the closer proximity of the nuclei and the greater concentration of electron density between them.
Bond Length
Shorter bond lengths generally correlate with stronger bonds and higher bond energies.
Examples Across Different Molecules
Bond energies vary considerably depending on the specific atoms involved and the molecular environment.
A few examples help illustrate this point:
- The bond energy of an O-H bond in water (H₂O) is approximately 467 kJ/mol.
- The bond energy of an H-H bond in hydrogen gas (H₂) is approximately 432 kJ/mol.
- The bond energy of a C-H bond in methane (CH₄) is approximately 413 kJ/mol.
- The bond energy of a Si-O bond in silicon dioxide (SiO₂) is approximately 452 kJ/mol.
Having explored the energies inherent within individual bonds, a crucial question arises: how do these bond energies influence the overall energy change observed in exothermic and endothermic reactions? The answer lies in the dynamic interplay between bond breaking and bond formation during a chemical transformation.
Bond Energy, Exothermic and Endothermic Reactions: A Tightly Knit Relationship
Chemical reactions are, at their core, a dance of bond breaking and bond formation. Reactants transform into products through the disruption of existing chemical bonds and the creation of new ones.
The energy required for and released by these processes dictates whether a reaction is exothermic or endothermic. It’s a tale of energetic investment versus energetic return.
The Energetic Balance Sheet
Every chemical reaction involves both breaking existing bonds within the reactants and forming new bonds to create the products. Each of these processes is associated with a specific energy change.
To understand whether a reaction will release or absorb energy overall, we must consider the relative amounts of energy involved in these two competing processes.
- Bond Breaking: Requires energy input.
- Bond Formation: Releases energy.
Exothermic Reactions: A Net Energy Release
In exothermic reactions, the energy released during the formation of new bonds in the products is greater than the energy required to break the bonds in the reactants. This surplus of released energy manifests as heat, which is then liberated into the surroundings.
Consider the combustion of methane (CH4). Breaking the bonds in methane and oxygen requires a certain amount of energy.
However, the formation of bonds in carbon dioxide (CO2) and water (H2O) releases significantly more energy. This net release of energy makes the reaction exothermic, producing a noticeable increase in temperature.
Endothermic Reactions: A Net Energy Input
Conversely, in endothermic reactions, the energy released during the formation of new chemical bonds in the products is less than the energy required to break the chemical bonds in the reactants.
This disparity creates an energy deficit, which must be supplied from the surroundings in the form of heat for the reaction to proceed.
Take, for example, the decomposition of water (H2O) into hydrogen (H2) and oxygen (O2). Breaking the strong bonds in water molecules requires a substantial amount of energy.
While the formation of bonds in hydrogen and oxygen molecules does release energy, it is insufficient to compensate for the initial energy investment.
This net energy requirement makes the reaction endothermic, causing a decrease in the temperature of the surroundings unless a continuous energy source is provided.
Implications of Bond Energy Differences
The difference in bond energies between reactants and products isn’t arbitrary. It’s directly linked to the stability of the molecules involved.
Exothermic reactions tend to form more stable products (lower energy state) than the reactants, leading to a net release of energy.
Endothermic reactions, on the other hand, result in products that are less stable (higher energy state) than the reactants, requiring a continuous energy input to maintain the reaction.
By analyzing bond energies, chemists can predict whether a reaction is likely to be exothermic or endothermic. This knowledge is crucial in designing and optimizing chemical processes for a wide variety of applications.
Having established the fundamental energy dynamics that differentiate exothermic and endothermic reactions, it’s time to explore the tangible manifestations of these processes in our everyday world. By examining concrete examples, we can further solidify our understanding of how these reactions operate and their profound impact on our surroundings.
Examples of Exothermic Reactions in Action
Exothermic reactions, characterized by the release of heat into the surroundings, are ubiquitous in both natural and artificial settings. These reactions power our homes, fuel our vehicles, and even drive critical biological processes. Let’s delve into some prominent examples to illustrate the diverse nature and significance of exothermic reactions.
Combustion of Fuels: A Fiery Release
Perhaps the most recognizable example of an exothermic reaction is combustion, the rapid reaction between a substance with an oxidant, usually oxygen, to produce heat and light.
The burning of wood in a fireplace, the combustion of propane in a grill, and the burning of natural gas in a furnace are all prime examples. In each case, the chemical bonds within the fuel molecules (wood, propane, or methane) are broken, and new bonds are formed between carbon and oxygen (to form carbon dioxide) and between hydrogen and oxygen (to form water).
These newly formed bonds are more stable and possess lower energy than the original bonds, leading to a net release of energy in the form of heat and light.
Neutralization Reactions: The Dance of Acids and Bases
Neutralization reactions, the reactions between acids and bases, are another common type of exothermic reaction. When an acid and a base are mixed, they react to form a salt and water.
This process releases heat, causing the temperature of the solution to increase.
For example, the reaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH) generates sodium chloride (NaCl) and water (H2O), along with a significant amount of heat.
This heat release is due to the formation of strong bonds in the water molecules and the formation of the ionic lattice in the salt, both of which are lower in energy than the original reactants.
Explosions: A Rapid Chain Reaction
Explosions represent a particularly dramatic and forceful type of exothermic reaction. In an explosion, a large amount of energy is released in a short period, creating a rapid expansion of volume. This expansion generates a shock wave that can cause significant damage.
Examples of explosive exothermic reactions include the detonation of dynamite, the ignition of fireworks, and the explosion of methane gas in a confined space.
In these reactions, unstable chemical compounds rapidly decompose, forming more stable products and releasing vast amounts of energy. The speed and magnitude of energy release differentiate explosions from other exothermic reactions. Fireworks, for instance, are carefully designed to control the rate of the explosion to produce spectacular visual displays.
The exothermic reactions detailed above are merely a few examples of the widespread prevalence and importance of heat-releasing reactions. Understanding these processes is paramount in numerous fields, from energy production to chemical synthesis and safety management.
Neutralization reactions demonstrate the heat-releasing power of exothermic processes, but the world is equally full of reactions that require an input of energy to occur. These endothermic reactions, characterized by the absorption of heat from their surroundings, are responsible for a wide array of phenomena, from the phase transitions of matter to the culinary delights we enjoy. Let’s examine a few familiar examples to illustrate the vital role of endothermic reactions in our lives.
Examples of Endothermic Reactions in Action
Endothermic reactions stand in stark contrast to their exothermic counterparts, pulling heat into the system from the surrounding environment. This absorption of energy results in a noticeable temperature drop in the immediate vicinity. Let’s explore some common examples that highlight the endothermic nature of these reactions.
Melting of Ice: A Classic Phase Transition
One of the most readily observable examples of an endothermic reaction is the melting of ice. In this process, solid water (ice) absorbs heat from its surroundings to transition into liquid water.
The heat energy is used to overcome the intermolecular forces holding the water molecules in a fixed crystalline structure. As the ice absorbs heat, the water molecules gain kinetic energy, vibrating more vigorously until they break free from their rigid positions.
This phase transition requires a significant input of energy, which is why a glass of ice water will cool down a room, as the ice draws heat from its surroundings to fuel the melting process. The temperature remains constant at 0°C (32°F) during the melting process, as the energy is used solely for the phase change, not for increasing the temperature.
Evaporation of Water: From Liquid to Gas
Similar to melting, the evaporation of water is an endothermic process that requires heat input. When liquid water transforms into gaseous water vapor, it absorbs energy from its surroundings.
This energy is used to overcome the attractive forces between water molecules in the liquid state, allowing them to escape into the air as a gas.
Consider the feeling of coolness after stepping out of a shower or swimming pool. As the water on your skin evaporates, it absorbs heat from your body, leading to a cooling sensation.
Evaporation plays a crucial role in regulating temperature in various natural processes, such as perspiration in animals and transpiration in plants.
Cooking: Baking a Cake: A Culinary Transformation
Many cooking processes involve endothermic reactions that transform raw ingredients into delicious dishes. Baking a cake, for instance, relies on heat absorption to drive various chemical reactions that alter the texture, flavor, and structure of the batter.
Deconstructing the Cake’s Chemistry
The heat from the oven provides the energy needed for several key endothermic reactions:
-
Starch gelatinization: Starch granules in the flour absorb water and swell, contributing to the cake’s structure.
-
Protein denaturation: Proteins in the eggs and flour unfold and coagulate, providing a solid framework for the cake.
-
Chemical leavening: Baking powder or baking soda decomposes, releasing gases that create air pockets and make the cake rise.
Each of these processes requires energy input in the form of heat and without a sustained supply of heat, the cake will not "bake" properly; it may remain a soggy, unappetizing mess. This makes baking a quintessential example of how endothermic reactions shape our everyday experiences.
Real-World Applications: Exothermic and Endothermic Reactions Everywhere
The principles governing exothermic and endothermic reactions aren’t confined to the laboratory. They are fundamental forces shaping a myriad of processes that define our industrial practices, daily existence, and even the biological systems within us. Understanding these reactions provides valuable insight into how we can manipulate energy flow to our advantage.
Exothermic and Endothermic Reactions in Industrial Chemistry
Industrial chemistry relies heavily on both types of reactions. Many industrial processes require a controlled release or absorption of heat to produce desired products efficiently.
Production of Ammonia (Haber-Bosch Process)
The Haber-Bosch process, a cornerstone of modern agriculture, is an exothermic reaction used to synthesize ammonia (NH3) from nitrogen and hydrogen. The released heat is carefully managed to maintain optimal reaction rates and efficiency. Without controlled management, the energy released could lead to dangerous temperature spikes.
Production of Plastics
The creation of many polymers also relies on exothermic polymerization reactions. The heat generated must be removed to prevent uncontrolled reactions and ensure the final product has the desired properties.
Metal Extraction
Conversely, some metal extraction processes rely on endothermic reactions to separate metals from their ores. This requires significant energy input, often in the form of heat, to drive the reaction forward.
Exothermic and Endothermic Reactions in Everyday Life
From the food we eat to the technology we use, exothermic and endothermic reactions play a vital role.
Cooking and Baking
Cooking is an excellent example of manipulating both types of reactions. Baking a cake is largely endothermic; heat is required to drive chemical changes like protein denaturation and starch gelatinization. Frying foods, on the other hand, often relies on exothermic reactions like oxidation, which contribute to browning and flavor development.
Heating and Cooling
Our homes rely on the application of these reactions to stay comfortable. Combustion of fuels like natural gas in furnaces is an exothermic reaction that provides heat. Refrigerators and air conditioners utilize endothermic processes involving the evaporation of refrigerants to absorb heat from the inside, cooling the space.
Instant Cold Packs
Instant cold packs use an endothermic reaction to provide immediate cooling. When the pack is activated, a salt like ammonium nitrate dissolves in water, absorbing heat from the surroundings and creating a cooling effect.
Exothermic and Endothermic Reactions in Biology
Life itself depends on carefully regulated exothermic and endothermic reactions. These reactions allow living systems to maintain energy balance and carry out essential functions.
Cellular Respiration
Cellular respiration, the process by which organisms convert food into energy, is an exothermic reaction. Glucose is oxidized, releasing energy that is stored in the form of ATP (adenosine triphosphate). This ATP then powers various cellular processes.
Photosynthesis
Photosynthesis, on the other hand, is an endothermic reaction. Plants absorb light energy from the sun to convert carbon dioxide and water into glucose and oxygen. This process is crucial for sustaining life on Earth.
Muscle Contraction
Muscle contraction involves both exothermic and endothermic processes. The breakdown of ATP to provide energy for muscle movement is exothermic. The subsequent rebuilding of ATP requires energy input, making it effectively endothermic.
In summary, exothermic and endothermic reactions are not merely academic concepts. They are ubiquitous, driving a wide range of processes that shape our industries, impact our daily lives, and sustain all biological systems. Understanding these fundamental reactions empowers us to innovate and control the world around us.
Calorimetry: Measuring the Invisible Heat
Having explored the multifaceted nature of exothermic and endothermic reactions and their real-world implications, it is important to delve into the methods scientists use to quantify the heat exchanged in these processes. This is where calorimetry comes into play, acting as a critical tool in the chemist’s arsenal.
Calorimetry is, at its core, the science of measuring heat.
More specifically, it is a technique used to determine the heat transferred during chemical reactions or physical changes.
Think of it as a precise thermometer, but one designed to capture and measure the total amount of heat released or absorbed by a reaction, rather than just a change in temperature.
The Calorimeter: A Heat-Measuring Device
The instrument used in calorimetry is called a calorimeter.
Different types of calorimeters exist, each designed for specific applications and levels of precision.
However, they all share a common principle: to isolate the reaction or process being studied and accurately measure the heat exchanged with the surroundings.
A simple calorimeter, often used in introductory chemistry labs, might consist of an insulated container, a thermometer, and a stirrer.
More sophisticated calorimeters, like bomb calorimeters, are designed to withstand high pressures and temperatures, making them suitable for studying combustion reactions.
How Calorimetry Works
The fundamental principle behind calorimetry rests on the conservation of energy.
The heat released or absorbed by the reaction (the system) is equal to the heat absorbed or released by the surroundings, typically the water in the calorimeter.
By carefully measuring the temperature change of the surroundings (the water), and knowing its mass and specific heat capacity, one can calculate the heat transferred during the reaction.
This calculation is typically done using the equation:
q = mcΔT
Where:
- q is the heat transferred
- m is the mass of the water
- c is the specific heat capacity of water
- ΔT is the change in temperature
Applications of Calorimetry
Calorimetry isn’t just a theoretical exercise; it has a wide range of practical applications.
It’s used extensively in:
- Determining the caloric content of food: Nutritional information on food labels relies on bomb calorimetry to measure the heat released when food is completely burned.
- Measuring the heat of combustion of fuels: This is crucial for evaluating the efficiency of different fuels.
- Studying the thermodynamics of chemical reactions: Calorimetry provides valuable data for determining enthalpy changes, reaction rates, and equilibrium constants.
- Pharmaceutical research: Determining the heat of solution of drugs is important for understanding their solubility and bioavailability.
In essence, calorimetry provides the quantitative data necessary to understand and control the energy dynamics of chemical and physical processes. It allows us to move beyond simply observing whether a reaction is exothermic or endothermic, and instead, to measure how much heat is involved. This is a critical step in advancing our understanding of chemistry and its applications.
Having seen how calorimetry allows us to quantify heat flow, the next logical question is: can we predict whether a reaction will happen on its own, without continuous external influence? The answer lies in the realm of Gibbs Free Energy.
Gibbs Free Energy: Predicting Spontaneity
Gibbs Free Energy (G), named after Josiah Willard Gibbs, is a thermodynamic potential that combines enthalpy (H) and entropy (S) to determine the spontaneity of a chemical reaction at a constant temperature (T) and pressure. In essence, it tells us whether a reaction will proceed forward without requiring continuous external energy input.
Defining Gibbs Free Energy
The Gibbs Free Energy is defined by the equation:
G = H – TS
Where:
-
G represents the Gibbs Free Energy.
-
H is the enthalpy of the system.
-
T is the absolute temperature (in Kelvin).
-
S is the entropy of the system.
Enthalpy (H) relates to the heat content of a system, while entropy (S) is a measure of the disorder or randomness within the system.
The Gibbs Free Energy essentially balances the drive towards lower energy (enthalpy) with the drive towards greater disorder (entropy).
The Significance of ΔG (Change in Gibbs Free Energy)
The change in Gibbs Free Energy (ΔG) during a reaction is the crucial factor in determining spontaneity.
It is calculated as:
ΔG = ΔH – TΔS
The sign of ΔG dictates whether a reaction is spontaneous (also called exergonic) or non-spontaneous (endergonic) at a given temperature:
-
ΔG < 0: Spontaneous Reaction:
The reaction will proceed forward on its own, without continuous external energy input. The reaction favors product formation. -
ΔG > 0: Non-Spontaneous Reaction:
The reaction will not proceed forward on its own. Continuous external energy input is required to drive the reaction forward. The reaction favors reactant retention. -
ΔG = 0: Reaction at Equilibrium:
The reaction is at equilibrium, meaning the rates of the forward and reverse reactions are equal. There is no net change in the concentrations of reactants and products.
Interpreting ΔG: A Balancing Act
A negative ΔG indicates that the decrease in enthalpy (ΔH) or the increase in entropy (ΔS), or both, are large enough to overcome any unfavorable contributions from the other.
Conversely, a positive ΔG suggests that the increase in enthalpy or the decrease in entropy is too significant for the reaction to proceed spontaneously.
It is essential to note that Gibbs Free Energy only predicts the thermodynamic favorability of a reaction. It does not provide any information about the rate at which the reaction will occur. A reaction may be spontaneous (ΔG < 0) but proceed extremely slowly due to kinetic factors, such as a high activation energy.
Temperature Dependence of Spontaneity
Temperature plays a critical role in determining spontaneity, particularly when both enthalpy and entropy changes are significant.
The TΔS term in the Gibbs Free Energy equation highlights this dependence.
For example, a reaction with a positive ΔH (endothermic) and a positive ΔS may be non-spontaneous at low temperatures but become spontaneous at higher temperatures, as the TΔS term becomes larger and more influential.
Conversely, a reaction with a negative ΔH (exothermic) and a negative ΔS may be spontaneous at low temperatures but become non-spontaneous at higher temperatures.
Gibbs Free Energy in Real-World Applications
The concept of Gibbs Free Energy is fundamental to various scientific and engineering disciplines.
It’s used:
-
In chemistry, to predict the feasibility of chemical reactions, design new reactions, and optimize reaction conditions.
-
In materials science, to understand phase transitions and predict the stability of different materials.
-
In biology, to analyze metabolic pathways and understand the energy requirements of living organisms.
Exothermic vs Endothermic Reactions: Your Burning Questions Answered
Here are some frequently asked questions to further clarify the fascinating differences between exothermic and endothermic reactions.
How can I easily tell if a reaction is exothermic vs endothermic?
The easiest way is to measure the temperature. Exothermic reactions release heat, causing the surroundings to become warmer. Endothermic reactions absorb heat, causing the surroundings to become cooler.
Does endothermic mean the reaction is slow?
Not necessarily. While endothermic reactions require energy input to proceed, the speed of the reaction depends on other factors like activation energy and catalysts, not just whether it’s exothermic vs endothermic. Some endothermic reactions can be quite fast if sufficient energy is supplied.
Are combustion reactions always exothermic?
Yes, combustion is a rapid chemical process involving heat and light. These reactions always release energy, making them definitively exothermic. It is a core characteristic of combustion.
Can a reaction be both exothermic and endothermic?
No, a reaction is classified as either exothermic or endothermic based on the net energy change. However, a complex reaction might involve multiple steps, some of which could be exothermic and others endothermic, but the overall reaction is either one or the other based on the overall energy released or absorbed.
Alright, there you have it – a hopefully not-too-scary dive into the world of exothermic vs endothermic reactions. Now you can confidently tell the difference between heat-releasing and heat-absorbing processes! Go forth and observe the world with your newfound knowledge!