Beryllium, an element within the alkaline earth metals group, exhibits a unique electronic structure. Understanding the electron configuration beryllium requires knowledge of quantum mechanics principles. The Aufbau principle dictates the filling order of electron orbitals, directly influencing beryllium’s chemical behavior. This configuration has important implications in various fields, from materials science, where beryllium’s properties are leveraged, to basic research in understanding atomic structure.
Beryllium (Be), a relatively rare element in the universe, holds a fascinating position within the periodic table.
Its unique combination of properties makes it both intriguing and essential to various fields of science and technology.
From its lightweight nature to its high melting point, Beryllium stands apart.
Understanding the arrangement of its electrons is paramount to unlocking its chemical behavior.
Setting the Stage: Why Beryllium?
This article aims to demystify the electron configuration of Beryllium.
We will explore the fundamental principles that govern how its electrons are arranged around the nucleus.
Electron configuration dictates how an element interacts with others, forms compounds, and participates in chemical reactions.
The Importance of Electron Configuration in Chemistry
Electron configuration is not just an abstract concept.
It is a cornerstone of understanding chemical reactivity and bonding.
The way electrons are arranged determines an element’s ability to form chemical bonds with other atoms, leading to the creation of molecules and compounds.
For example, elements with similar electron configurations often exhibit similar chemical behaviors, a concept neatly organized in the periodic table.
Understanding electron configurations allows us to predict and explain these trends.
This knowledge is crucial for designing new materials, understanding biological processes, and developing new technologies.
The way electrons are arranged profoundly affects how elements behave, but to grasp this concept fully, we must first establish a firm understanding of atomic structure. This provides the bedrock upon which the principles of electron configuration are built. Let’s explore the fundamental components of an atom and how they relate to Beryllium.
Atomic Foundation: Understanding Beryllium’s Atomic Structure
The Atom’s Core: Nucleus and Electron Cloud
At the heart of every element lies the atom. The atom itself is composed of two primary regions: the nucleus and the electron cloud.
The nucleus, a dense and positively charged core, contains protons and neutrons. Protons carry a positive charge, while neutrons are neutral, contributing to the atom’s mass but not its charge.
Surrounding the nucleus is the electron cloud, a vast region where negatively charged electrons reside. This cloud is not a solid structure but rather a probabilistic area where electrons are most likely to be found.
Electrons are constantly in motion around the nucleus, held in place by the electromagnetic force of attraction to the positively charged protons.
Atomic Number: The Element’s Identity
The atomic number is a fundamental property of an element. It defines the number of protons found within the nucleus of every atom of that element.
The atomic number is unique to each element and serves as its identifier within the periodic table.
In a neutral atom, the number of protons is equal to the number of electrons. This balance ensures that the overall charge of the atom is zero.
Therefore, the atomic number also indicates the number of electrons present in a neutral atom of that element.
Beryllium’s Atomic Number: 4
Beryllium (Be) has an atomic number of 4. This signifies that every Beryllium atom contains 4 protons within its nucleus.
Consequently, a neutral Beryllium atom also possesses 4 electrons orbiting the nucleus.
This relatively small number of electrons makes Beryllium a manageable starting point for understanding electron configuration.
The arrangement of these 4 electrons determines Beryllium’s chemical behavior and its interactions with other elements.
The previous section established the fundamental atomic architecture of Beryllium, revealing its composition of protons, neutrons, and electrons. Understanding these basic building blocks allows us to transition into an exploration of how these electrons arrange themselves around the nucleus. It is in this arrangement that an element’s true character is revealed.
The Crucial Role of Electron Configuration
Electron configuration is more than just a description of where electrons reside; it’s a powerful tool for understanding and predicting chemical behavior. This arrangement dictates an element’s interactions with other elements, its bonding capabilities, and even its physical properties.
Defining Electron Configuration
Electron configuration describes the specific arrangement of electrons within an atom. It details which energy levels and sublevels are occupied by electrons. This arrangement follows specific rules dictated by quantum mechanics.
This detailed ‘address’ of each electron is crucial. It allows us to predict how an atom will interact with other atoms to form molecules.
The Link to Chemical Properties and Reactivity
The electron configuration is the direct determinant of an element’s chemical properties. It dictates how an element will react with others.
Valence electrons, those in the outermost shell, are the key players in chemical bonding.
An element’s drive to achieve a stable electron configuration (typically resembling a noble gas) dictates its reactivity. Elements will gain, lose, or share electrons to achieve this stability.
For example, elements with nearly full outer shells tend to gain electrons, while those with only a few tend to lose them. This drive leads to the formation of chemical bonds.
Electron Configuration and the Periodic Table
The periodic table is not just a list of elements. It’s an organized chart reflecting recurring patterns in electron configurations.
Elements within the same group (vertical column) share similar valence electron configurations. They exhibit similar chemical behaviors.
The periodic table allows us to predict electron configurations based on an element’s position. This significantly simplifies understanding its properties. The table is essentially a map of electronic structures.
Trends in ionization energy, electronegativity, and atomic size are all directly related to electron configuration and its periodic variations. Understanding electron configuration unlocks the secrets of the periodic table. It transforms it from a mere chart into a powerful predictive tool.
The previous section established the fundamental atomic architecture of Beryllium, revealing its composition of protons, neutrons, and electrons. Understanding these basic building blocks allows us to transition into an exploration of how these electrons arrange themselves around the nucleus. It is in this arrangement that an element’s true character is revealed.
Orbitals Demystified: A Guide to s, p, d, and f
Electron configuration relies on understanding the concept of atomic orbitals.
These aren’t physical pathways electrons follow, but rather mathematical descriptions of regions where an electron is likely to be found.
Think of them as probability maps, charting the electron’s most probable locations around the nucleus.
The Shapes of Orbitals
There are four principal types of atomic orbitals, designated as s, p, d, and f.
Each has a distinctive shape and spatial orientation.
s orbitals are the simplest, possessing a spherical shape centered around the nucleus.
Each energy level has one s orbital.
p orbitals are dumbbell-shaped and exist in sets of three, oriented along the x, y, and z axes.
This gives them directionality in space.
d orbitals are more complex, with most having four lobes and existing in sets of five.
Their shapes are significantly more intricate than s and p orbitals.
f orbitals are the most complex of the common orbitals, possessing complicated multi-lobed shapes.
They exist in sets of seven.
Filling Order and Energy Levels
Electrons fill orbitals in a specific order, dictated by their energy levels.
Lower energy levels are filled before higher ones.
The filling order generally follows this sequence: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p, and so on.
Notice that the 4s orbital fills before the 3d orbitals, despite the general rule. This is due to subtle energy differences between the sublevels.
The Guiding Principles: Aufbau, Hund’s, and Pauli
While we won’t delve into extensive detail here, it’s important to acknowledge the principles governing orbital filling.
These rules provide a framework for correctly predicting electron configurations.
The Aufbau Principle states that electrons first occupy the lowest energy orbitals available.
It’s a ‘bottom-up’ approach to filling orbitals.
Hund’s Rule dictates that electrons will individually occupy each orbital within a subshell before doubling up in any one orbital.
This maximizes overall spin.
The Pauli Exclusion Principle states that no two electrons in an atom can have the same set of four quantum numbers.
This means that each orbital can hold a maximum of two electrons, and they must have opposite spins.
These principles, in conjunction with an understanding of orbital shapes and energy levels, are crucial for accurately determining the electron configurations of elements.
They ensure the stability and predictability of atomic behavior.
The filling order of orbitals can seem abstract, but understanding it is key to deciphering an element’s electron configuration. Let’s now apply these principles to Beryllium itself. The result is not just a string of numbers and letters, but a fundamental description of its electronic structure.
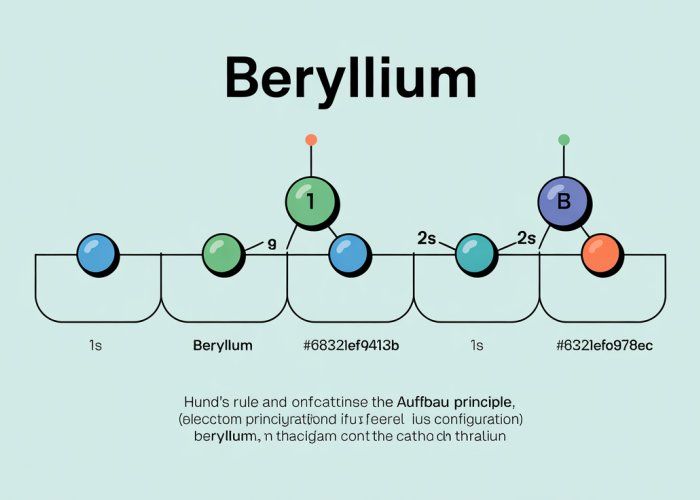
Beryllium’s Configuration: A Step-by-Step Breakdown (1s²2s²)
To accurately represent Beryllium’s electron configuration, we need to carefully follow the filling order of atomic orbitals based on their energy levels. This process reveals the specific arrangement of its four electrons.
Deciphering the Electron Configuration of Beryllium
Beryllium has an atomic number of 4.
This dictates that a neutral Beryllium atom contains four electrons.
These electrons must be strategically placed within the available atomic orbitals, adhering to the principles we’ve discussed.
The filling process is as follows:
-
The 1s Orbital: The 1s orbital is the lowest energy orbital and can hold a maximum of two electrons. Therefore, the first two electrons of Beryllium fill this orbital completely, resulting in the notation 1s².
-
The 2s Orbital: Once the 1s orbital is filled, the next two electrons proceed to occupy the 2s orbital. The 2s orbital can also hold a maximum of two electrons. Beryllium’s remaining two electrons completely fill the 2s orbital, resulting in the notation 2s².
Combining these two steps, we arrive at the complete electron configuration for Beryllium: 1s²2s².
Filling Order and Beryllium’s Simplicity
Beryllium’s relatively low atomic number simplifies the electron configuration process.
Elements with more electrons require filling higher energy orbitals, leading to more complex configurations.
However, with only four electrons, Beryllium’s configuration is straightforward, involving only the 1s and 2s orbitals.
This simplicity provides a clear illustration of the fundamental principles guiding electron configuration.
Understanding Beryllium’s configuration serves as an excellent foundation for tackling more complex elements later on.
The Significance of the Filled 2s Orbital
The 2s orbital in Beryllium is completely filled.
This seemingly small detail holds significant implications for Beryllium’s chemical behavior.
Filled orbitals represent a state of relative stability.
While Beryllium isn’t entirely inert, the filled 2s orbital contributes to its tendency to form covalent bonds rather than readily losing or gaining electrons like alkali or halogen elements.
This characteristic defines much of Beryllium’s chemistry.
The complete electron configuration, 1s²2s², and the filled 2s orbital, explain, in part, why Beryllium behaves the way it does in chemical reactions.
Beryllium’s relatively low atomic number simplifies the electron configuration process. Elements with more electrons require filling higher energy orbitals, leading to more complex configurations and, often, more varied chemical behaviors. Now, let’s consider how this specific arrangement of electrons influences Beryllium’s interactions with other elements, focusing on the critical role of valence electrons.
Valence Electrons: Beryllium’s Key to Reactivity (or Lack Thereof)
Defining Valence Electrons
Valence electrons are the electrons residing in the outermost electron shell of an atom. These are the electrons primarily responsible for chemical bonding, as they are the ones that interact with other atoms.
Understanding valence electrons is fundamental to predicting how an element will react and form compounds. Atoms tend to gain, lose, or share valence electrons to achieve a stable electron configuration, often resembling that of a noble gas (octet rule).
Identifying Beryllium’s Valence Electrons
Beryllium, with its electron configuration of 1s²2s², has two valence electrons. These two electrons reside in the 2s orbital, which is the outermost shell for Beryllium.
It is these two electrons that dictate Beryllium’s bonding behavior.
Beryllium’s Reactivity: A Unique Case
While many elements strive to achieve a full outer shell (8 electrons), Beryllium presents an interesting case.
It has a relatively high ionization energy (the energy required to remove an electron). It has a tendency to form covalent bonds rather than readily losing its two valence electrons to form ionic bonds.
Beryllium’s small size and relatively high charge density contribute to this behavior.
Reactivity Considerations
Beryllium’s reactivity is not as straightforward as some other elements in the same group. While it can form compounds, it often does so under specific conditions.
In some forms, Beryllium exhibits a lack of reactivity due to the formation of a protective oxide layer on its surface, hindering further reactions.
Beryllium compounds are known to be toxic and must be handled with care. This further limits their applications and the extent to which Beryllium participates in reactions.
Now that we’ve dissected the mechanics of Beryllium’s electron configuration and explored how valence electrons dictate its behavior, it’s time to move from abstract notation to a more tangible representation. Visualizing the electron configuration provides a spatial understanding that can solidify the concepts discussed so far.
Visualizing the Configuration: A Diagrammatic Representation
Electron configurations, while informative, can sometimes feel abstract. A visual aid, specifically an orbital diagram, can bridge the gap between notation and understanding, making the spatial arrangement of electrons far more intuitive.
The Power of Visual Representation
Diagrams offer a powerful way to reinforce learning. They translate complex information into a format that is more easily processed and retained. For electron configurations, this means representing the arrangement of electrons within their respective orbitals in a clear, concise manner.
Constructing Beryllium’s Orbital Diagram
To effectively illustrate Beryllium’s electron configuration (1s²2s²), we’ll use a standard orbital diagram format:
- Boxes or circles representing each orbital (1s and 2s in Beryllium’s case).
- Arrows within the boxes to represent electrons, with the direction of the arrow indicating electron spin (either spin-up or spin-down).
Orbital Arrangement for Beryllium
-
1s Orbital: This is represented by a single box. It contains two electrons, depicted as one upward-pointing arrow and one downward-pointing arrow. This illustrates that the 1s orbital is fully occupied.
-
2s Orbital: Similar to the 1s orbital, the 2s orbital is also represented by a single box. It too contains two electrons, one with spin-up and one with spin-down. This completes Beryllium’s electron configuration.
The Importance of Clear Labeling
For maximum clarity, each orbital should be clearly labeled (1s, 2s). Additionally, each electron should be represented with distinct arrows, differentiating them by spin.
This visual clarity is essential to avoid confusion.
Interpreting the Diagram
The completed diagram showcases several key aspects of Beryllium’s electron configuration:
- The number of electrons in each orbital is immediately apparent.
- The paired spin of electrons within each orbital is visually emphasized, reinforcing the Pauli Exclusion Principle.
- The relative energy levels of the orbitals are implicitly represented by their position in the diagram (1s being lower in energy than 2s).
Benefits of Using a Diagram
Using a diagrammatic representation of Beryllium’s electron configuration offers several advantages:
- Improved Comprehension: The visual format makes it easier to grasp the spatial arrangement of electrons.
- Enhanced Retention: Visual aids improve memory retention compared to purely textual information.
- Clearer Understanding of Electron Spin: Arrows clearly illustrate the concept of electron spin and its role in filling orbitals.
By translating the abstract notation of electron configurations into a visual format, we create a more engaging and accessible learning experience. An orbital diagram provides a tangible representation that enhances comprehension and solidifies understanding of Beryllium’s electron structure.
Now that we’ve built a solid visual understanding of Beryllium’s electron configuration, it’s crucial to address some common pitfalls that can trip up even seasoned chemistry students. Recognizing and avoiding these misconceptions is key to mastering electron configurations for all elements.
Avoiding Pitfalls: Common Misconceptions and Errors
Electron configurations, while governed by clear rules, are often a source of confusion. Many students encounter similar challenges when first learning about them. By addressing these common misconceptions head-on and providing practical tips, we can ensure a more solid and confident understanding.
The Confusing World of Orbital Filling Order
One of the most frequent errors stems from misunderstanding the order in which electrons fill the orbitals. It’s not as simple as just going straight from 1s to 2s to 2p and so on.
The energy levels of orbitals can sometimes overlap. This means a higher n number orbital can have lower energy than another orbital with a lower n.
For example, the 4s orbital fills before the 3d orbital. This is because, despite having a higher principal quantum number, the 4s orbital is slightly lower in energy than the 3d orbital.
Using the Aufbau principle and the (n+l) rule can help to correctly predict the filling order. The Aufbau principle states that electrons first fill the lowest energy orbitals available. The (n+l) rule dictates that the lower the sum of n (principal quantum number) and l (azimuthal quantum number), the lower the energy of the orbital.
Visual aids, such as the Madelung rule diagram, can be extremely helpful in memorizing and applying the correct filling order. It’s not about rote memorization, but about understanding the energetic principles at play.
Ignoring Hund’s Rule: The Importance of Electron Pairing
Hund’s Rule states that within a subshell (p, d, or f), electrons will individually occupy each orbital before any orbital is doubly occupied, and that all electrons in singly occupied orbitals have the same spin (are parallel).
A common mistake is to pair electrons in an orbital before filling all the orbitals within a subshell with single electrons. For example, in the 2p subshell (which has three orbitals), you must place one electron in each of the three 2p orbitals before adding a second electron to any of them.
This minimizes electron-electron repulsion and results in a more stable configuration. Remember to apply Hund’s Rule whenever dealing with p, d, and f orbitals.
The Pauli Exclusion Principle: No Two Electrons Alike
The Pauli Exclusion Principle states that no two electrons in an atom can have the same set of four quantum numbers. This translates practically to meaning that each orbital can hold a maximum of two electrons, and those electrons must have opposite spins (+1/2 and -1/2).
Forgetting this principle leads to incorrect electron configurations with more than two electrons in a single orbital. Always double-check that each orbital is filled according to this fundamental rule.
Overlooking Exceptions to the Rules
While the Aufbau principle, Hund’s rule, and Pauli exclusion principle provide a strong foundation, there are exceptions. Chromium (Cr) and Copper (Cu) are classic examples.
Chromium, instead of having a configuration of [Ar] 4s² 3d⁴, adopts [Ar] 4s¹ 3d⁵. This is because a half-filled d subshell (d⁵) is more stable than a partially filled d subshell with a filled s subshell.
Similarly, Copper shifts from [Ar] 4s² 3d⁹ to [Ar] 4s¹ 3d¹⁰ to achieve a full d subshell (d¹⁰), which is also more stable. Be aware of these exceptions and understand the driving force behind them: the pursuit of increased stability.
Tips for Error-Free Electron Configurations
To minimize mistakes and build confidence, consider these strategies:
- Practice Regularly: The more electron configurations you write, the more comfortable and proficient you’ll become.
- Use a Periodic Table as a Guide: The periodic table is structured based on electron configurations. Use its organization to predict the filling order.
- Double-Check Your Work: Always review your completed electron configurations to ensure they adhere to the rules and principles discussed.
- Seek Feedback: Ask a teacher, tutor, or classmate to review your work and provide constructive criticism.
- Understand, Don’t Just Memorize: Focus on understanding why the rules exist, not just memorizing them. This will make it easier to apply them correctly in different situations.
By addressing these common misconceptions and implementing these helpful tips, you can confidently navigate the intricacies of electron configurations and build a stronger foundation in chemistry.
FAQs: Beryllium’s Electron Configuration
Want to deepen your understanding of beryllium and its electron structure? Check out these frequently asked questions.
What exactly does electron configuration describe?
Electron configuration describes how electrons are arranged within the different energy levels and sublevels of an atom. For example, the electron configuration of beryllium shows which orbitals are occupied by its electrons. It’s like a map of where the electrons "live."
What is beryllium’s electron configuration?
The electron configuration of beryllium is 1s²2s². This means it has two electrons in the 1s orbital (the innermost shell) and two electrons in the 2s orbital. Understanding this configuration is key to grasping beryllium’s chemical behavior.
How does beryllium’s electron configuration relate to its position on the periodic table?
Beryllium (Be) is in Group 2 (alkaline earth metals) and Period 2 of the periodic table. The period number tells you the highest energy level occupied by electrons, which is 2 for beryllium. The electron configuration beryllium also ends in s², which is characteristic of Group 2 elements.
Why is understanding electron configuration important for beryllium?
Understanding electron configuration is important because it dictates how beryllium interacts with other atoms and forms chemical bonds. Beryllium’s tendency to lose its two valence electrons from the 2s orbital allows it to form stable compounds. This is crucial for predicting and explaining its chemical properties.
So there you have it – a peek into the world of electron configuration beryllium! Hopefully, this makes the whole concept a little clearer. Now go forth and explore the fascinating world of chemistry!