Understanding muscle contraction is fundamental to comprehending human movement and athletic performance. Actin and myosin filaments, key components of muscle fibers, generate force through the crossbridge cycle. This process, heavily researched by scientists at institutions like the National Institutes of Health (NIH), describes how muscle fibers shorten and produce movement. ATP, the energy currency of the cell, is essential for powering the cycle and enabling muscles to function properly. The crossbridge cycle, therefore, is the cornerstone to unlock muscle power.
Unlocking the Secrets of Muscle Movement
From the simple act of blinking to the complex coordination required for athletic feats, muscle movement is fundamental to nearly every aspect of our lives.
Consider the intricate dance of muscles involved in maintaining posture, allowing us to sit upright or stand tall.
Think about the delicate precision of muscles controlling our eyes as we read these words.
Or, reflect on the sheer power and coordinated action of muscles propelling a sprinter across the finish line.
These diverse actions, seemingly disparate, are all powered by a single, elegant mechanism: the crossbridge cycle.
The Core of Contraction: The Crossbridge Cycle
At the heart of every muscle contraction, from the subtlest twitch to the most forceful exertion, lies the crossbridge cycle.
This cyclical process, occurring at the molecular level, is the engine that drives muscle fiber shortening and, consequently, movement.
Understanding the crossbridge cycle is therefore crucial for comprehending not only how our bodies move, but also the underlying causes of various muscle-related conditions and diseases.
A Roadmap to Muscle Mechanics
This exploration will delve into the intricate world of the crossbridge cycle, unraveling its complexities and highlighting its vital role in human physiology.
We’ll begin by introducing the key players – the molecules and structures – that participate in this microscopic dance.
Next, we will break down the cycle into its distinct stages.
Each step will reveal the molecular events that allow muscles to contract and generate force.
We will also examine the regulatory role of calcium, the crucial ion that triggers and controls muscle contraction.
Finally, we will explore the processes involved in muscle relaxation, explaining how the crossbridge cycle is reversed to allow muscles to lengthen and rest.
Unraveling the elegant dance of the crossbridge cycle requires familiarity with its key players. Before we can delve into the cycle’s mechanics, it’s essential to understand the roles and characteristics of the molecules and structures that orchestrate muscle contraction. Consider this section your introduction to the cast of characters, where each element plays a vital part in this microscopic ballet.
Meet the Players: Key Components of the Crossbridge Cycle
The crossbridge cycle, the very essence of muscle contraction, is a fascinating interplay of various molecular components. To truly grasp the mechanics of this cycle, one must first become acquainted with its principal actors. These include the structural proteins actin and myosin, the energy currency ATP, the regulatory gatekeepers calcium ions, troponin, and tropomyosin, and the fundamental unit, the sarcomere. Each element plays a distinctive and indispensable role in this intricate process.
Actin: The Filamentous Foundation
Actin is a globular protein that polymerizes to form long, filamentous structures called actin filaments, or thin filaments. These filaments serve as the track upon which myosin motors move, driving muscle contraction.
Each actin monomer contains a binding site for myosin, the motor protein of muscle. However, under resting conditions, these binding sites are often blocked by regulatory proteins, preventing premature or uncontrolled contraction.
Myosin: The Molecular Motor
Myosin is a protein that forms the thick filaments within muscle fibers. Each myosin molecule consists of a long tail and a globular head. The myosin head is the critical region responsible for interacting with actin and generating force.
This head contains an ATP-binding site and an actin-binding site. Through a cycle of ATP hydrolysis and conformational changes, the myosin head binds to actin, pulls the thin filament, and then detaches, ready to repeat the process.
ATP (Adenosine Triphosphate): The Energy Currency
ATP, or Adenosine Triphosphate, is the primary source of energy for the crossbridge cycle. It fuels the conformational changes in the myosin head that drive muscle contraction.
The energy released from ATP hydrolysis (the breaking of a phosphate bond) is harnessed to "cock" the myosin head into a high-energy state, ready to bind to actin. Following the power stroke, another ATP molecule is required for myosin to detach from actin, resetting the cycle.
ADP (Adenosine Diphosphate) and Phosphate (Pi): Byproducts of Energy Expenditure
ADP (Adenosine Diphosphate) and inorganic phosphate (Pi) are the products of ATP hydrolysis. The release of these byproducts from the myosin head triggers the power stroke, the actual force-generating step of the crossbridge cycle.
The binding affinity of myosin for actin changes upon the release of Pi and ADP, contributing to the contraction process. Understanding these byproducts highlights the energetic demands of muscle contraction.
Calcium Ions (Ca2+): The Trigger for Contraction
Calcium ions (Ca2+) act as the crucial trigger for initiating the crossbridge cycle. In a resting muscle, the concentration of calcium ions in the sarcoplasm (the cytoplasm of muscle cells) is very low.
When a nerve impulse reaches the muscle, it triggers the release of calcium ions from the sarcoplasmic reticulum, an intracellular calcium store. These calcium ions then bind to troponin, initiating the chain of events leading to muscle contraction.
Troponin and Tropomyosin: The Gatekeepers of Actin
Troponin and tropomyosin are regulatory proteins that control the interaction between actin and myosin. Tropomyosin is a long, thin molecule that wraps around the actin filament, physically blocking the myosin-binding sites.
Troponin is a complex of three proteins (Troponin I, Troponin T, and Troponin C) that binds to tropomyosin. When calcium ions bind to Troponin C, it causes a conformational change that shifts tropomyosin away from the myosin-binding sites on actin, allowing crossbridge formation.
Sarcomere: The Functional Unit of Muscle
The sarcomere is the basic contractile unit of muscle. It is the repeating segment of myofibrils, the long cylindrical structures that run the length of muscle fibers.
The sarcomere is defined by the region between two Z-lines and contains both actin (thin) and myosin (thick) filaments. The shortening of the sarcomere, driven by the crossbridge cycle, results in muscle contraction.
Sliding Filament Theory: The Big Picture
The sliding filament theory describes how muscle contraction occurs through the sliding of actin and myosin filaments relative to each other. This sliding is powered by the crossbridge cycle.
As myosin heads repeatedly bind to actin, pull the thin filaments towards the center of the sarcomere, detach, and repeat, the sarcomere shortens. Numerous sarcomeres shortening simultaneously throughout the muscle fiber result in macroscopic muscle contraction.
Unraveling the elegant dance of the crossbridge cycle requires familiarity with its key players. Before we can delve into the cycle’s mechanics, it’s essential to understand the roles and characteristics of the molecules and structures that orchestrate muscle contraction. Consider this section your introduction to the cast of characters, where each element plays a vital part in this microscopic ballet.
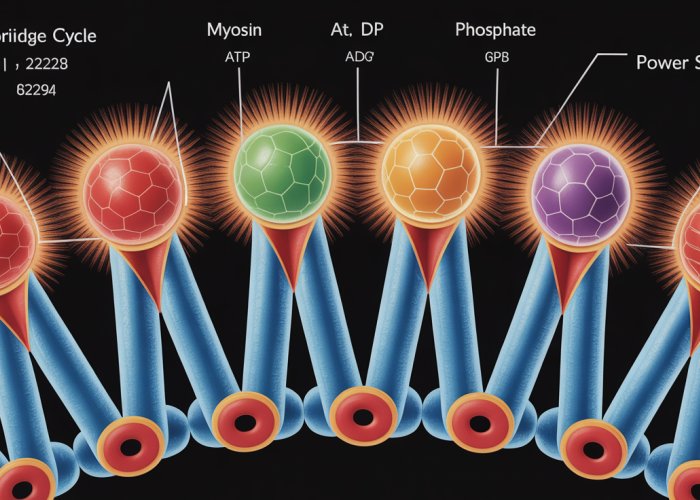
The Crossbridge Cycle: A Step-by-Step Guide
Now that we’ve met the key players, it’s time to choreograph their movements. The crossbridge cycle, the engine of muscle contraction, is a recurring sequence of events that converts chemical energy into mechanical work. Let’s break down this intricate process into its core stages, revealing the molecular events that occur at each critical juncture.
Calcium Ion Binding: The Ignition Switch
The entire process hinges on the presence of calcium ions (Ca2+).
In a relaxed muscle, the binding sites on actin are blocked by the troponin-tropomyosin complex, preventing myosin from attaching.
When a nerve impulse arrives, it triggers the release of calcium ions from the sarcoplasmic reticulum.
These calcium ions then bind to troponin, causing a conformational change that shifts tropomyosin away from the actin binding sites, effectively "unlocking" actin.
Myosin Head Binding: Forming the Crossbridge
With actin’s binding sites exposed, the myosin head, already energized by ATP hydrolysis (as we’ll see later), can now attach.
This attachment forms a crossbridge, the physical link between the thick and thin filaments.
The strength of this bond is crucial for force generation.
The Power Stroke: The Engine of Contraction
The power stroke is the pivotal event where the myosin head pivots, pulling the actin filament towards the center of the sarcomere.
This movement is powered by the release of ADP and inorganic phosphate (Pi) from the myosin head.
It’s during this stroke that the actual muscle shortening occurs. The force generated is proportional to the number of crossbridges engaged.
ATP Binding: Detachment and Reset
Once the power stroke is complete, a new molecule of ATP binds to the myosin head.
This binding causes a conformational change in the myosin head, weakening its affinity for actin and resulting in detachment of the crossbridge.
This detachment is essential for the cycle to continue; otherwise, the myosin head would remain bound, leading to muscle stiffness.
ATP Hydrolysis: Re-Energizing the Myosin Head
The ATP bound to the myosin head is then hydrolyzed (broken down) into ADP and inorganic phosphate (Pi).
This hydrolysis re-energizes the myosin head, returning it to its "cocked" or high-energy conformation.
The ADP and Pi remain bound to the myosin head, poised to be released during the next power stroke, provided calcium ions are still present and the actin binding sites are exposed.
This continuous cycle of attachment, power stroke, detachment, and re-energizing drives the sliding of actin filaments along myosin filaments, resulting in muscle contraction. Understanding each step is critical to appreciating the elegant efficiency of this fundamental biological process.
Now that we’ve seen the cycle in motion, let’s rewind and zoom in on the crucial spark that ignites the entire process: calcium. Its presence, or absence, dictates whether our muscles contract or remain at rest. This seemingly simple ion wields immense power over our ability to move.
Calcium’s Crucial Role: Initiating Muscle Contraction
Calcium ions (Ca2+) are the gatekeepers of muscle contraction. They act as the primary signal, the on/off switch that determines whether the crossbridge cycle can even begin.
Without calcium, the entire machinery of muscle contraction remains idle.
The Trigger for Contraction
The story begins with a nerve impulse reaching the muscle fiber. This triggers the release of calcium ions from the sarcoplasmic reticulum, an internal storage network within muscle cells.
These calcium ions flood the sarcoplasm, the cytoplasm of the muscle cell.
The influx of calcium is the critical event that sets the stage for the crossbridge cycle.
Unlocking Actin’s Binding Sites
As we discussed earlier, in a relaxed muscle, the binding sites on actin filaments are physically blocked by the troponin-tropomyosin complex. Myosin heads cannot attach, and no force can be generated.
Calcium ions, however, have a high affinity for troponin. When calcium binds to troponin, it induces a conformational change in the troponin-tropomyosin complex.
This shift effectively "unlocks" the actin binding sites, exposing them for myosin to bind.
The unveiling of these binding sites is the crucial step that allows the crossbridge cycle to commence. Without calcium, this unveiling cannot occur.
The Regulatory Function
The role of calcium extends beyond simply initiating the crossbridge cycle. It also serves as a regulator, dictating the strength and duration of the contraction.
The more calcium ions that are present and bound to troponin, the more binding sites on actin are exposed.
This, in turn, allows more myosin heads to attach and generate force.
Conversely, as calcium levels decrease, fewer binding sites are available. Fewer crossbridges form, and the force of contraction diminishes.
Absence of Calcium: Preventing Contraction
The absence of calcium is just as crucial as its presence. When calcium levels in the sarcoplasm are low, the troponin-tropomyosin complex resumes its blocking position, covering the myosin-binding sites on actin.
This prevents myosin from attaching, effectively shutting down the crossbridge cycle.
This is the default state of a relaxed muscle: low intracellular calcium levels, blocked actin binding sites, and no crossbridge formation.
This constant blocking by the troponin-tropomyosin complex ensures that muscles remain relaxed and do not contract involuntarily. This precise control is essential for coordinated movement and preventing muscle spasms.
The dependence of muscle contraction on calcium highlights the exquisite control mechanisms that govern our movements. It is a delicate balance, where the presence or absence of a single ion can determine whether we can walk, run, or even breathe.
Now that we’ve seen the cycle in motion, and the crucial spark that ignites the entire process with calcium, we should examine how the contraction ceases. Muscle contraction isn’t a permanent state; our muscles need to relax to allow for subsequent movements and prevent fatigue. Understanding the relaxation process is just as important as understanding the contraction itself, as it involves a carefully orchestrated series of events that reverse the steps of the crossbridge cycle.
Relaxation: Reversing the Contraction
Muscle relaxation is an active process, not simply the absence of stimulation. It requires energy and the coordinated action of several cellular mechanisms to undo the events of muscle contraction. This reversal is essential for proper muscle function and overall body movement.
The Crucial Role of Calcium Removal
The first and perhaps most critical step in muscle relaxation is the removal of calcium ions (Ca2+) from the sarcoplasm. This process effectively shuts down the signal that initiates and sustains the crossbridge cycle.
Calcium Reuptake by the Sarcoplasmic Reticulum
Specialized protein pumps, known as calcium ATPases, are located in the membrane of the sarcoplasmic reticulum. These pumps actively transport calcium ions back into the sarcoplasmic reticulum, reducing the calcium concentration in the sarcoplasm.
This pumping action requires ATP, highlighting the energy-dependent nature of muscle relaxation. As calcium levels fall, the stage is set for the next phase of relaxation.
Restoring the Blockade: Troponin and Tropomyosin Return
With calcium ions being actively sequestered back into the sarcoplasmic reticulum, the calcium bound to troponin starts to dissociate. This dissociation triggers a chain of events that leads to the repositioning of troponin and tropomyosin.
As calcium detaches, troponin reverts to its original conformation, allowing tropomyosin to slide back into its blocking position over the actin binding sites. This physical blockade prevents myosin heads from attaching to actin, effectively stopping the crossbridge cycle.
Myosin Detachment and Sarcomere Lengthening
The final stages of relaxation involve the detachment of myosin from actin and the return of the sarcomere to its resting length. With the actin binding sites now covered, myosin heads can no longer form strong attachments.
As ATP binds to the myosin head, the crossbridge weakens, and the myosin detaches from actin. This detachment is critical for allowing the muscle to relax.
Passive Sarcomere Lengthening
With the crossbridges broken and no active force being generated, the sarcomere returns to its resting length. This lengthening is aided by the elastic properties of the muscle fibers and the opposing forces of antagonistic muscles.
Connective tissues within the muscle also contribute to this passive lengthening, ensuring the muscle is ready for the next contraction. Thus, the muscle relaxes and awaits the next signal for contraction, showcasing the intricate dance of molecular events that govern our movement.
Clinical Relevance: When Things Go Wrong
Now that we’ve examined the mechanics of relaxation, it’s crucial to consider the broader biological context and the implications of crossbridge cycle dysfunction. Muscle contraction, while seemingly simple, is a finely tuned process susceptible to disruption at various points. Understanding these potential points of failure is critical for diagnosing and treating a range of neuromuscular disorders.
The Neuromuscular Junction: Where Signals Begin
The journey of muscle contraction begins not within the muscle fiber itself, but at the neuromuscular junction (NMJ). This specialized synapse is where a motor neuron communicates with a muscle fiber, initiating the cascade of events that ultimately lead to the crossbridge cycle.
At the NMJ, the motor neuron releases a neurotransmitter called acetylcholine (ACh).
ACh diffuses across the synaptic cleft and binds to ACh receptors on the muscle fiber membrane (the sarcolemma).
This binding depolarizes the sarcolemma, creating an action potential that propagates along the muscle fiber.
Disruptions at the NMJ can have devastating consequences.
For example, in myasthenia gravis, an autoimmune disorder, the body produces antibodies that attack ACh receptors.
This reduces the number of available receptors, impairing the muscle fiber’s ability to respond to ACh.
The result is muscle weakness and fatigue, particularly in muscles controlling eye movement, facial expression, and swallowing.
Excitation-Contraction Coupling: Linking Nerve Signals to Muscle Action
The action potential generated at the NMJ doesn’t directly trigger the crossbridge cycle. Instead, it initiates a process called excitation-contraction coupling. This intricate mechanism translates the electrical signal from the nerve into a chemical signal that can stimulate muscle contraction.
The action potential travels along the sarcolemma and into the T-tubules, invaginations of the sarcolemma that penetrate deep into the muscle fiber.
T-tubules are closely associated with the sarcoplasmic reticulum (SR), an intracellular store of calcium ions.
When the action potential reaches the T-tubules, it activates voltage-sensitive receptors called dihydropyridine receptors (DHPRs).
DHPRs are mechanically linked to ryanodine receptors (RyRs), which are calcium channels located on the SR membrane.
Activation of DHPRs causes RyRs to open, releasing a flood of calcium ions into the sarcoplasm.
This surge in calcium concentration is what triggers the crossbridge cycle, as we discussed earlier.
Defects in excitation-contraction coupling can lead to various muscle disorders.
For example, malignant hyperthermia is a rare but life-threatening condition triggered by certain anesthetic drugs.
In susceptible individuals, these drugs cause uncontrolled release of calcium from the SR, leading to sustained muscle contraction, a rapid increase in body temperature, and potentially death.
Mutations in RyR1, the gene encoding the ryanodine receptor in skeletal muscle, are often responsible for malignant hyperthermia.
Understanding the intricacies of the neuromuscular junction and excitation-contraction coupling is crucial for understanding a wide range of neuromuscular disorders. By pinpointing the specific steps that are disrupted in these conditions, researchers can develop targeted therapies to restore normal muscle function.
FAQs: The Crossbridge Cycle
Here are some common questions about the crossbridge cycle and how it contributes to muscle power.
What exactly is the crossbridge cycle?
The crossbridge cycle is a series of molecular events that occur when a muscle fiber contracts. It involves the interaction of actin and myosin filaments, where myosin heads bind to actin, pull it, and then detach. This cycle repeats continuously, shortening the muscle.
Why is the crossbridge cycle so important for muscle power?
Because the crossbridge cycle is what generates the force that drives muscle contraction. Each cycle contributes a small amount of force. The more cycles that occur, and the faster they occur, the greater the force and therefore, the more power the muscle generates.
What role does ATP play in the crossbridge cycle?
ATP (adenosine triphosphate) is crucial. It provides the energy needed for the myosin head to detach from the actin filament after the power stroke. Without ATP, the myosin head remains bound, leading to muscle rigidity (rigor mortis). ATP also powers the repositioning of the myosin head to begin the crossbridge cycle again.
How does calcium affect the crossbridge cycle?
Calcium ions are essential for initiating muscle contraction. They bind to troponin, which causes tropomyosin to shift away from the myosin-binding sites on actin. This exposure allows the myosin heads to attach to actin and start the crossbridge cycle. Without calcium, this process cannot begin.
So, there you have it – the crossbridge cycle demystified! Hope this helped you understand how your muscles actually *move* you. Now go out there and put that knowledge to good use!