The kidney’s function, a vital process for maintaining homeostasis, heavily relies on the intricate countercurrent mechanism. This mechanism, particularly active within the Loop of Henle, is instrumental in establishing the medullary osmotic gradient. The medullary osmotic gradient, an important concept in nephrology, allows for the concentration of urine and the conservation of water. Understanding the nuances of the countercurrent mechanism kidney provides a critical insight into renal physiology and its impact on overall health. This mechanism allows the vasa recta to supply nutrients and remove waste products without disturbing the osmotic gradient.
We’ve all been there: that moment of intense thirst after a workout, a long day in the sun, or simply forgetting to drink enough water.
While reaching for that refreshing glass of water feels intuitive, do you ever stop to think about what happens after you quench your thirst?
The real magic of hydration isn’t just in the drinking, but in how your body manages that fluid. And at the heart of this intricate system are your kidneys.
The Silent Guardians of Fluid Balance
The kidneys, often overlooked, are essential for maintaining the delicate balance of fluids and electrolytes in your body.
These bean-shaped organs work tirelessly, filtering waste and excess water from your blood, ensuring that your cells function optimally.
They’re not just filters, though. They’re sophisticated regulators, carefully adjusting the composition of your urine to maintain the perfect internal environment.
The Countercurrent Mechanism: A Marvel of Engineering
How do the kidneys achieve this remarkable feat of fluid conservation? The answer lies in a brilliant process called the countercurrent mechanism.
This intricate system allows your kidneys to efficiently reabsorb water, preventing dehydration and ensuring that vital nutrients aren’t flushed away.
The countercurrent mechanism is a crucial component in maintaining homeostasis.
What We’ll Explore
In this article, we’ll delve into the fascinating world of the countercurrent mechanism and its vital role in kidney function. We’ll explore:
- The anatomy of the kidney and the nephron, its functional unit.
- How the Loop of Henle creates an osmolarity gradient.
- The role of the vasa recta in preserving this gradient.
- The influence of hormones like ADH on water reabsorption.
- Clinical implications when this system malfunctions.
By understanding the countercurrent mechanism, you’ll gain a newfound appreciation for the silent, yet critical work your kidneys perform every single day.
Your Kidneys and Nephrons: An Inside Look
Before we can fully appreciate the intricacies of the countercurrent mechanism, it’s essential to understand the landscape in which it operates: the kidneys themselves and their microscopic functional units, the nephrons. These structures are the key players in maintaining fluid balance, and their specific anatomy directly enables the remarkable water conservation capabilities of the kidneys.
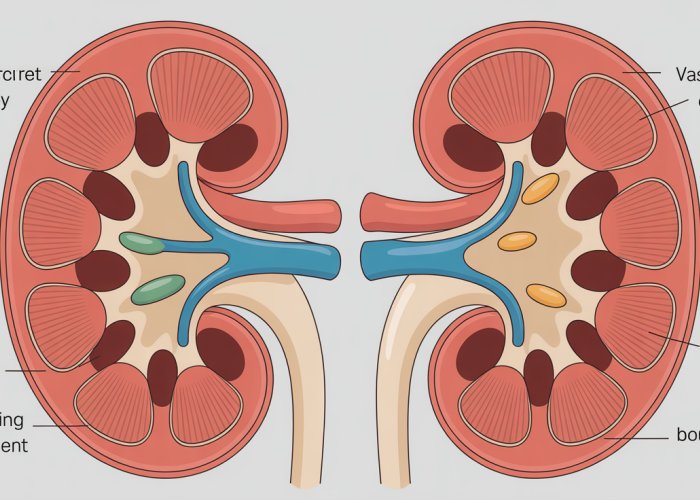
Kidney Anatomy: A Layered Approach
The kidneys, two bean-shaped organs located in the abdominal cavity, exhibit a distinct layered structure. The two primary regions are the cortex and the medulla.
The cortex, the outer layer, is where the initial filtration of blood occurs. It’s a highly vascularized region, teeming with nephrons, the tiny filtering units.
The medulla, the inner region, is characterized by its conical structures called renal pyramids. These pyramids are primarily composed of the Loops of Henle and collecting ducts of the nephrons, the very structures responsible for concentrating urine. The arrangement of these structures in the medulla is critical for establishing the osmotic gradient necessary for water reabsorption.
The Nephron: The Functional Unit
The nephron is the workhorse of the kidney, the smallest unit capable of performing the essential functions of filtration, reabsorption, and secretion. Each kidney contains approximately one million nephrons, each diligently processing blood to produce urine.
Understanding the nephron’s structure is paramount to grasping the countercurrent mechanism.
Nephron Structure: A Detailed Overview
The nephron is composed of several distinct sections:
-
Glomerulus: This is a network of capillaries where blood is initially filtered. High pressure forces water and small solutes out of the blood and into Bowman’s capsule, the next structure in line.
-
Proximal Tubule: Here, the majority of reabsorption occurs. Essential substances like glucose, amino acids, sodium, and water are transported back into the bloodstream.
-
Loop of Henle: This is a U-shaped structure that dips into the medulla. Its descending and ascending limbs play a crucial role in establishing the osmotic gradient that drives water reabsorption.
-
Distal Tubule: This segment is responsible for further reabsorption and secretion, fine-tuning the electrolyte and acid-base balance of the filtrate.
-
Collecting Duct: This is the final segment of the nephron, where urine concentration is regulated under the influence of hormones like ADH (antidiuretic hormone). Multiple nephrons drain into a single collecting duct, which then empties into the renal pelvis.
The Loop of Henle’s Significance
Among all the nephron’s components, the Loop of Henle stands out as particularly important for the countercurrent mechanism. Its unique structure and permeability characteristics create the osmotic gradient within the medulla, enabling the kidneys to produce concentrated urine and conserve water. The descending limb is permeable to water but not to ions, while the ascending limb is permeable to ions but not to water. This difference in permeability is what establishes the concentration gradient that allows the body to reclaim water.
Your kidneys, with their intricate nephron networks, are primed for their most impressive feat: concentrating urine and reclaiming water. Let’s delve into the engine that drives this process – the countercurrent mechanism.
The Countercurrent Mechanism: How Your Kidneys Conserve Water
At the heart of the kidney’s water-saving prowess lies the countercurrent mechanism. This sophisticated system, operating within the nephrons, orchestrates a delicate interplay of fluid flow and solute movement to produce concentrated urine. It’s not merely a passive process; it’s an active, energy-intensive operation crucial for maintaining fluid balance.
What is the Countercurrent Mechanism?
The countercurrent mechanism is, in essence, a system where fluid flows in opposite directions in two adjacent limbs. In the kidney, these limbs are the descending and ascending limbs of the Loop of Henle.
The primary purpose of this mechanism is two-fold:
-
Concentrating urine: By creating a hyperosmotic environment in the kidney’s medulla, the countercurrent mechanism allows water to be drawn out of the urine in the collecting ducts, resulting in a more concentrated final product.
-
Efficient water reabsorption: This concentrated medullary environment acts as a powerful osmotic force, pulling water back into the bloodstream and preventing excessive water loss.
Without this remarkable mechanism, we would lose a significant amount of water through urine, leading to dehydration and electrolyte imbalances.
The Role of the Loop of Henle
The Loop of Henle, a hairpin-shaped structure within the nephron, is the central stage for the countercurrent mechanism. Its unique architecture and varying permeabilities in its descending and ascending limbs are essential for establishing the osmotic gradient necessary for water reabsorption.
Descending Limb: Water Permeability
The descending limb of the Loop of Henle is highly permeable to water but relatively impermeable to solutes. As the filtrate (the fluid filtered from the blood) flows down this limb, it encounters the increasingly concentrated environment of the kidney’s medulla.
This concentration gradient draws water out of the filtrate via osmosis, further concentrating the filtrate as it descends deeper into the medulla.
Ascending Limb: Ion Permeability
In contrast to the descending limb, the ascending limb is impermeable to water but actively transports ions, primarily sodium (Na+) and chloride (Cl-), out of the filtrate and into the surrounding medullary tissue.
This active transport of ions contributes to the increasing concentration of the medulla while simultaneously diluting the filtrate as it ascends towards the cortex.
Osmolarity Gradient Creation
The contrasting permeabilities of the descending and ascending limbs, coupled with the active transport of ions, are the key to creating the osmolarity gradient in the medulla. This gradient, ranging from approximately 300 mOsm/L in the cortex to 1200 mOsm/L in the deepest part of the medulla, is the driving force behind water reabsorption.
The gradient gets steeper as you go deeper into the medulla.
The Importance of the Medulla on the Countercurrent Mechanism
The medulla is not simply a passive recipient of the processes occurring within the Loop of Henle. Its unique structural organization and composition are crucial for the proper functioning of the countercurrent mechanism.
The arrangement of the Loops of Henle and collecting ducts within the medulla ensures that the filtrate is exposed to a progressively increasing osmotic concentration as it travels through the kidney. This controlled exposure allows for the fine-tuning of water reabsorption and urine concentration.
Furthermore, the presence of specialized cells and transport proteins within the medulla facilitates the active transport of ions and the maintenance of the osmotic gradient. The medulla, therefore, acts as a critical facilitator and regulator of the countercurrent mechanism, ensuring efficient water conservation.
The Loop of Henle masterfully establishes the osmotic gradient, but its existence depends on another key player: the vasa recta. These specialized capillaries work in tandem with the loop to prevent the dissipation of this carefully crafted gradient.
The Vasa Recta: Preserving the Delicate Balance
The vasa recta are a network of specialized peritubular capillaries that intimately associate with the Loop of Henle within the kidney’s medulla.
Their unique structure and arrangement are critical for maintaining the hyperosmotic environment necessary for concentrating urine. They act as countercurrent exchangers, preventing the washout of solutes from the medulla.
Parallel Architecture: A Design for Preservation
The vasa recta follow a hairpin-shaped path, running parallel and in close proximity to the Loops of Henle.
This parallel arrangement is not coincidental; it’s crucial for their function as countercurrent exchangers.
As the vasa recta descend into the medulla, they encounter increasing osmolarity, mirroring the environment experienced by the descending limb of the Loop of Henle.
Conversely, as they ascend back towards the cortex, they pass through regions of decreasing osmolarity, similar to the ascending limb.
Countercurrent Exchange: Preventing Gradient Dissipation
The primary function of the vasa recta is to prevent the dissipation of the medullary osmotic gradient, not to create it.
This is achieved through a process called countercurrent exchange.
As blood flows down the descending limb of the vasa recta, it encounters the increasingly hyperosmotic medullary interstitium.
Water moves out of the blood and into the interstitium, while solutes (like NaCl and urea) move from the interstitium into the blood.
This process concentrates the blood within the descending vasa recta.
As the blood ascends in the ascending limb of the vasa recta, it encounters decreasing osmolarity in the medullary interstitium.
The concentrated blood now releases solutes back into the interstitium and gains water.
The net result is that the vasa recta carry away water and a small amount of reabsorbed solutes, preventing the washout of the high osmolarity environment from the medulla.
The countercurrent exchange mechanism of the vasa recta is remarkably efficient. It removes water without drastically reducing the solute concentration in the medulla.
Maintaining the Medullary Environment
Without the vasa recta, blood flow through the medulla would quickly dissipate the osmotic gradient established by the Loop of Henle.
The vasa recta’s architecture and countercurrent exchange mechanism ensure that the high solute concentration in the medulla is maintained. This allows for efficient water reabsorption in the collecting ducts.
This intricate interplay between the Loop of Henle and the vasa recta demonstrates the sophistication of the kidney’s water conservation system.
Their cooperation is essential for maintaining fluid balance and overall homeostasis.
The precise choreography within the kidney, involving the Loop of Henle and the vasa recta, establishes and safeguards the osmotic gradient critical for water conservation. However, the story doesn’t end there. The body has a hormonal messenger that acts as a precise regulator of water reabsorption, ensuring we maintain the right balance even when external conditions fluctuate.
ADH: The Hormonal Conductor of Water Reabsorption
Antidiuretic Hormone (ADH), also known as vasopressin, is a key player in the intricate process of water reabsorption within the kidneys. This hormone acts as a conductor, fine-tuning the collecting duct’s permeability to water based on the body’s hydration needs. Without ADH, the carefully established medullary gradient would be far less effective at drawing water back into the bloodstream.
ADH’s Influence on Collecting Duct Permeability
The collecting duct, the final segment of the nephron, is the primary site of ADH action. In the absence of ADH, the collecting duct’s walls are relatively impermeable to water. This means that the filtrate flowing through it continues on to be excreted as dilute urine.
However, when the body detects dehydration, the pituitary gland releases ADH into the bloodstream. ADH then travels to the kidneys and binds to receptors on the cells lining the collecting duct.
This binding triggers a cascade of intracellular events, ultimately leading to the insertion of aquaporins—water channel proteins—into the apical membrane of these cells.
Aquaporins act like tiny doorways, allowing water to move freely across the cell membrane, following the osmotic gradient established by the countercurrent mechanism. The more ADH present, the more aquaporins are inserted, and the more permeable the collecting duct becomes to water.
Enhanced Reabsorption with the Osmolarity Gradient
The effectiveness of ADH is inextricably linked to the medullary osmolarity gradient. The hyperosmotic environment of the medulla, created by the Loop of Henle and maintained by the vasa recta, provides the driving force for water reabsorption.
As the filtrate flows through the collecting duct, the high solute concentration in the surrounding medullary interstitium draws water out of the filtrate, through the aquaporins, and back into the bloodstream.
This process concentrates the urine, reducing water loss and helping to maintain fluid balance.
The stronger the medullary gradient, the more water can be reabsorbed under the influence of ADH. In states of severe dehydration, when ADH levels are high and the medullary gradient is robust, the kidneys can produce highly concentrated urine, minimizing water loss.
Impact on Nephron Function
ADH’s influence extends beyond simply increasing water permeability in the collecting duct. By modulating water reabsorption, ADH indirectly impacts several other aspects of nephron function.
For example, it affects the concentration of solutes in the urine. When ADH promotes water reabsorption, the remaining solutes in the filtrate become more concentrated, affecting the excretion of waste products and electrolytes.
Furthermore, ADH interacts with other hormonal systems involved in fluid and electrolyte balance, such as the renin-angiotensin-aldosterone system (RAAS). These interactions ensure that water reabsorption is tightly regulated in response to various physiological signals.
Ultimately, ADH plays a critical role in maintaining homeostasis, ensuring that the body has the right amount of water to function optimally. Dysregulation of ADH can lead to a variety of clinical problems, highlighting its importance in overall health.
The body relies on hormonal regulation to fine-tune water reabsorption, ensuring that the delicate osmotic balance maintained within the kidneys isn’t easily disrupted. But the establishment and preservation of that medullary gradient – the engine driving water conservation – isn’t solely reliant on the architecture of the Loop of Henle and the precise countercurrent exchange of the vasa recta. Two key players, often working behind the scenes, are vital contributors: urea and sodium chloride.
Urea and Sodium Chloride: Essential Contributors to the Gradient
While the Loop of Henle and the vasa recta establish the architecture for water reabsorption, the true workhorses in creating the concentration gradient are two simple molecules: urea and sodium chloride (NaCl). These substances, through their unique transport mechanisms, supercharge the kidney’s ability to draw water back into the bloodstream, allowing us to conserve water and produce concentrated urine.
The Role of Urea
Urea, a waste product of protein metabolism, often gets a bad rap. However, in the kidney, it plays a surprisingly crucial role in establishing the medullary osmotic gradient.
Its contribution centers around a clever process known as urea recycling.
Urea Recycling: A Second Chance for Osmolarity
Urea, filtered out in the glomerulus, is initially present in the filtrate flowing through the nephron. As the filtrate moves through the collecting duct, which traverses the increasingly concentrated medulla, urea is transported into the medullary interstitium.
This movement is facilitated by specific urea transporters (UT-A1 and UT-A3) located in the collecting duct cells.
By increasing the urea concentration in the medulla, urea directly contributes to the overall osmolarity, making the environment even more hypertonic.
From Medulla Back to the Loop: The Cycle Continues
The beauty of urea recycling lies in its cyclical nature. From the medullary interstitium, urea diffuses into the thin ascending limb of the Loop of Henle.
This reinjection of urea into the loop ensures a continuous supply of this osmolyte, further amplifying the gradient. This recycling process isn’t perfectly efficient; some urea is inevitably excreted in the urine. However, the majority is cleverly repurposed to bolster the kidney’s concentrating power.
The Role of Sodium Chloride (NaCl)
Sodium chloride, common table salt, is another essential component of the medullary gradient. Unlike urea, which is passively transported in certain segments, NaCl is actively pumped out of the ascending limb of the Loop of Henle.
Active Transport in the Ascending Limb
The thick ascending limb is impermeable to water but actively transports Na+ and Cl- ions into the medullary interstitium. This active transport is mediated by the Na-K-2Cl cotransporter (NKCC2) located in the apical membrane of the tubule cells.
This transporter uses the energy of ATP to move one sodium ion, one potassium ion, and two chloride ions from the filtrate into the cell, and then into the interstitium.
By actively removing these ions from the filtrate, the ascending limb not only dilutes the fluid within the tubule but also significantly increases the osmolarity of the surrounding medullary tissue.
NaCl’s Contribution to the Medullary Gradient
The NaCl pumped into the interstitium contributes directly to the high osmolarity of the medulla. This increased osmolarity is what drives the reabsorption of water from the descending limb of the Loop of Henle and the collecting duct.
Without the active transport of NaCl in the ascending limb, the medullary gradient would be significantly weaker.
Consequently, the kidneys’ ability to concentrate urine and conserve water would be severely compromised.
When the System Fails: Clinical Implications of a Faulty Countercurrent Mechanism
The elegant precision of the countercurrent mechanism, while robust, is not invincible. Various conditions can disrupt this finely tuned system, leading to significant imbalances in fluid and electrolyte homeostasis. Understanding these clinical implications is crucial for recognizing and addressing potential kidney-related issues.
Conditions Disrupting the Countercurrent Mechanism
Several factors can impair the kidney’s ability to concentrate urine effectively. These disruptions often stem from hormonal imbalances, structural damage to the kidneys, or interference with the transport processes within the nephron.
-
Diabetes Insipidus (DI): This condition, characterized by the inability to concentrate urine, directly undermines the countercurrent mechanism. Central DI arises from a deficiency in ADH production or release, hindering the collecting duct’s permeability to water. Nephrogenic DI, on the other hand, involves the kidneys’ unresponsiveness to ADH, preventing water reabsorption despite adequate hormone levels.
-
Loop Diuretics: These medications, commonly prescribed for hypertension and edema, inhibit the Na+/K+/2Cl- cotransporter in the ascending limb of the Loop of Henle. By reducing sodium chloride reabsorption, loop diuretics diminish the medullary osmotic gradient, thereby reducing the kidneys’ concentrating ability.
-
Renal Tubular Damage: Conditions like acute tubular necrosis (ATN) or chronic kidney disease (CKD) can directly damage the nephron’s structure, especially the Loop of Henle and collecting ducts. This structural damage impairs the establishment and maintenance of the medullary gradient, resulting in reduced water reabsorption.
-
Sickle Cell Disease: In individuals with sickle cell disease, the sickled red blood cells can damage the vasa recta. The impaired blood flow disrupts the countercurrent exchange system, leading to a less concentrated medullary gradient.
Consequences of Impaired Water Reabsorption
When the countercurrent mechanism falters, the body struggles to conserve water, leading to a cascade of physiological consequences.
-
Polyuria and Polydipsia: The hallmark symptoms of a failing countercurrent mechanism are excessive urine production (polyuria) and increased thirst (polydipsia). The kidneys, unable to concentrate urine effectively, excrete large volumes of dilute urine. This loss of water triggers a powerful thirst response.
-
Dehydration: Chronic polyuria can lead to dehydration, especially if fluid intake cannot keep pace with water loss. Dehydration can manifest as dry mouth, dizziness, fatigue, and, in severe cases, altered mental status.
-
Electrolyte Imbalances: Impaired water reabsorption often disrupts electrolyte balance. Specifically, hyponatremia (low sodium levels) can occur due to excessive water loss relative to sodium. Hypernatremia (high sodium levels) can occur if water losses are not replaced. These imbalances can affect neurological function, muscle contraction, and cardiac rhythm.
-
Acid-Base Disturbances: Kidney dysfunction can affect the body’s ability to regulate acid-base balance. For example, renal tubular acidosis (RTA) involves impaired reabsorption of bicarbonate or excretion of acid, leading to metabolic acidosis.
-
Impact on Blood Pressure: The kidneys play a critical role in blood pressure regulation. Impaired water reabsorption can affect blood volume, which in turn impacts blood pressure control. Dehydration may lead to hypotension (low blood pressure), while sodium imbalances can contribute to hypertension (high blood pressure).
Understanding the clinical implications of a faulty countercurrent mechanism highlights the critical role this process plays in maintaining overall health. By recognizing the conditions that can disrupt this system and the subsequent consequences, healthcare professionals can better diagnose and manage kidney-related disorders, thereby promoting optimal fluid and electrolyte balance.
Conditions that impair the countercurrent mechanism reveal its importance, but the good news is that we can actively support this system. Simple lifestyle adjustments can significantly influence kidney health and the efficiency of this crucial water-retaining process. Understanding how our daily habits affect our kidneys empowers us to make informed choices that promote optimal fluid balance.
Keeping Your Kidneys Happy: Supporting a Healthy Countercurrent Mechanism
Our kidneys work tirelessly to maintain fluid balance, and supporting their function is paramount for overall health. The countercurrent mechanism, as we’ve explored, is central to this process. Fortunately, we can actively contribute to the health of our kidneys and optimize this crucial mechanism through conscious lifestyle choices.
The Cornerstone: Adequate Hydration
Hydration is perhaps the single most important factor in supporting a healthy countercurrent mechanism. When the body is adequately hydrated, the kidneys can efficiently concentrate urine, minimizing water loss.
Chronic dehydration, on the other hand, forces the kidneys to work harder, potentially impairing their ability to establish and maintain the medullary osmotic gradient.
Aim for a consistent intake of water throughout the day, and be mindful of increased needs during exercise or in hot weather.
Listen to your body’s signals and drink before you feel excessively thirsty. Individual hydration needs can vary based on activity level, climate, and overall health.
Lifestyle Factors and Kidney Function
Beyond hydration, several other lifestyle factors can significantly influence the health and efficiency of the countercurrent mechanism.
Diet’s Direct Impact
Diet plays a key role in kidney health. A diet high in sodium can increase the workload on the kidneys, as they must excrete the excess sodium.
Conversely, a balanced diet rich in fruits, vegetables, and whole grains provides essential nutrients that support overall kidney function.
Limiting processed foods, which are often high in sodium and unhealthy fats, is also beneficial. Adequate potassium intake is also crucial, as it helps regulate electrolyte balance and blood pressure.
Exercise and Blood Flow
Regular physical activity promotes healthy blood flow to the kidneys, ensuring they receive the oxygen and nutrients they need to function optimally.
Exercise also helps regulate blood pressure and blood sugar levels, which are important for preventing kidney damage.
However, it’s important to stay hydrated during exercise to compensate for fluid loss through sweat.
Medications and Prudent Use
Certain medications, particularly NSAIDs (nonsteroidal anti-inflammatory drugs), can be harmful to the kidneys if used excessively or long-term.
These medications can reduce blood flow to the kidneys and impair their ability to filter waste products.
Always consult with a healthcare professional before taking any medication regularly, especially if you have pre-existing kidney conditions.
Alcohol and Moderation
Excessive alcohol consumption can dehydrate the body and strain the kidneys. Alcohol also interferes with the release of ADH, which further impairs the kidneys’ ability to concentrate urine.
Moderation is key. If you choose to drink alcohol, do so in moderation and be sure to stay hydrated by drinking plenty of water.
By prioritizing adequate hydration, maintaining a healthy diet, engaging in regular physical activity, and being mindful of medication and alcohol consumption, we can actively support our kidneys and the vital countercurrent mechanism, ensuring optimal fluid balance and overall well-being.
FAQs: Understanding the Countercurrent Mechanism in Your Kidneys
[The countercurrent mechanism is vital for kidney function. Here are some frequently asked questions to help you understand it better.]
Why is the countercurrent mechanism important for kidney function?
The countercurrent mechanism kidney is essential for concentrating urine. Without it, your kidneys wouldn’t be able to reabsorb enough water, leading to dehydration. This process ensures we maintain the right balance of fluids in our body.
What exactly does "countercurrent" mean in this context?
"Countercurrent" refers to the flow of fluid in opposite directions within the loops of Henle and the vasa recta. This opposing flow allows for efficient exchange of solutes and water, maximizing the concentration gradient in the kidney.
Where does the countercurrent mechanism kidney primarily occur?
The countercurrent mechanism takes place in the nephrons of the kidneys, specifically within the loop of Henle and the surrounding blood vessels called the vasa recta. This specialized arrangement allows for the efficient concentration of urine.
How does the countercurrent mechanism help save water?
By establishing a high concentration gradient in the medulla (inner region) of the kidney, the countercurrent mechanism kidney allows the collecting ducts to reabsorb more water. This results in more concentrated urine and less water loss from the body.
So, now you’ve got a handle on the countercurrent mechanism kidney! Hopefully, this makes you appreciate your kidneys a little more. Keep drinking water and take care of those filters!