Understanding cellular metabolism equation is fundamental to grasping energy production within biological systems. Glycolysis, a crucial metabolic pathway, initiates the breakdown of glucose, yielding pyruvate as a key intermediate. The Mitochondria, often called the powerhouse of the cell, are the location where many vital steps happen, including the citric acid cycle and oxidative phosphorylation. Researchers at the National Institutes of Health (NIH) conduct extensive studies on cellular processes, including the intricate details of metabolic pathways and the cellular metabolism equation. Scientists use techniques like Mass Spectrometry to analyze metabolites and understand how the cellular metabolism equation is regulated in various organisms.
Life, in all its complexity, hinges on a fundamental principle: the intricate dance of chemical reactions occurring within cells. This symphony of processes is what we call cellular metabolism.
It’s not merely a biological process; it is the very essence of how cells obtain energy, synthesize essential molecules, and eliminate waste.
Understanding cellular metabolism, particularly through its central equation, unlocks insights into a vast range of biological phenomena. From the smallest microbe to the largest mammal, the principles remain constant.
Defining Cellular Metabolism
At its core, cellular metabolism is the complete set of chemical reactions that occur within a cell. These reactions are organized into metabolic pathways, where one chemical is transformed through a series of steps into another, by a sequence of enzymes.
These pathways are not random; they are highly regulated and coordinated to meet the cell’s ever-changing needs.
This includes everything from breaking down nutrients for energy to building complex molecules like proteins and DNA.
Think of it as the cell’s internal factory, constantly working to maintain life.
The Significance of the Cellular Metabolism Equation
The cellular metabolism equation provides a concise representation of these complex processes. It allows us to visualize the inputs and outputs of cellular energy production.
By understanding this equation, we can better grasp how cells obtain and utilize energy, as well as how they synthesize the molecules necessary for growth, repair, and other essential functions.
The equation is not just an academic exercise; it has far-reaching implications across diverse fields.
In medicine, it helps us understand metabolic disorders like diabetes and obesity.
In biotechnology, it informs the development of new drugs and therapies.
In sports science, it provides insights into optimizing athletic performance.
A Brief Overview of Key Components and Processes
The cellular metabolism equation, in its most generalized form, can be represented as:
Fuel + Oxygen → Carbon Dioxide + Water + Energy (ATP)
While this equation provides a high-level overview, the actual processes involved are far more complex. It involves several key components:
-
Fuel: Typically glucose, but can also include other carbohydrates, fats, and proteins.
-
Oxygen: Essential for efficient energy production in most organisms (aerobic respiration).
-
Carbon Dioxide: A waste product of cellular metabolism.
-
Water: Both a reactant and a product in various metabolic reactions.
-
ATP (Adenosine Triphosphate): The primary energy currency of the cell.
These components interact through a series of interconnected pathways, including:
-
Glycolysis: The breakdown of glucose into pyruvate.
-
The Krebs Cycle (Citric Acid Cycle): A series of reactions that extract energy from pyruvate.
-
The Electron Transport Chain: A process that uses electrons to generate a large amount of ATP.
Understanding these components and processes is crucial for deciphering the intricacies of cellular metabolism and its role in sustaining life.
Life’s processes are deeply intertwined with the intricate world of cellular metabolism. Understanding this fundamental principle opens doors to comprehending how cells function and sustain life. Now that we’ve established a clear definition and grasp the significance of cellular metabolism, it’s time to dissect the core equation that governs this vital process.
The Core Equation: A Detailed Breakdown
At the heart of cellular metabolism lies a fundamental equation that encapsulates the essence of energy production and molecular synthesis within cells. This generalized equation, while simplified, provides a powerful framework for understanding the inputs and outputs of cellular energy transformations.
Unveiling the Generalized Equation
The generalized equation representing cellular metabolism can be expressed as:
Glucose + Oxygen → Carbon Dioxide + Water + ATP
This equation signifies that in the presence of oxygen, cells break down glucose (a simple sugar) to produce carbon dioxide, water, and, most importantly, ATP (adenosine triphosphate).
Let’s dissect this equation further:
- Reactants: On the left side, we have the reactants, the substances that are consumed in the process. Glucose serves as the primary fuel source, while oxygen acts as the electron acceptor.
- Products: On the right side, we find the products, the substances generated as a result of the reaction. Carbon dioxide and water are waste products, while ATP is the energy currency that powers cellular activities.
The exact equation can vary depending on the specific metabolic pathway and the type of molecule being metabolized (e.g., fats or proteins). This core equation provides a foundational understanding of energy flow within cells.
The Central Role of ATP: The Cell’s Energy Currency
The cellular metabolism equation highlights the crucial role of ATP. ATP (adenosine triphosphate) is often referred to as the "energy currency" of the cell.
Why is ATP so important? It is the primary molecule that cells use to store and transport energy for various cellular processes.
Think of ATP as the cell’s rechargeable battery. When cells require energy to perform work, such as muscle contraction, protein synthesis, or active transport, they break down ATP, releasing the stored energy.
This released energy then fuels the specific cellular process.
The breakdown of ATP generates ADP (adenosine diphosphate) and inorganic phosphate.
ADP can then be recycled back into ATP through cellular metabolism, effectively "recharging" the battery. Without a constant supply of ATP, cells would quickly run out of energy and be unable to perform the functions necessary for life.
Life’s processes are deeply intertwined with the intricate world of cellular metabolism. Understanding this fundamental principle opens doors to comprehending how cells function and sustain life. Now that we’ve established a clear definition and grasp the significance of cellular metabolism, it’s time to dissect the core equation that governs this vital process.
Key Players: The Molecules of Metabolism
The cellular metabolism equation, while seemingly simple, involves a cast of crucial molecular players. Each molecule contributes uniquely to the overall process, ensuring efficient energy production and the synthesis of necessary cellular components. Understanding the specific roles of these molecules is key to appreciating the elegance and complexity of metabolic pathways.
Glucose: The Primary Fuel Source
Glucose, a simple sugar with the chemical formula C6H12O6, stands as the primary fuel source for cellular metabolism. Its relatively high energy content and ease of breakdown make it an ideal choice for cells.
Through a series of carefully orchestrated reactions, cells extract the energy stored within glucose molecules. This process releases energy to produce ATP, the energy currency of the cell.
Oxygen: The Essential Electron Acceptor
Oxygen plays a critical role, specifically in cellular respiration, as the final electron acceptor in the electron transport chain. This role is vital in efficiently generating a significant amount of ATP. Without oxygen, cells must resort to less efficient anaerobic pathways, such as fermentation.
The presence of oxygen allows for the complete oxidation of glucose, maximizing energy extraction. This is why aerobic organisms are capable of sustained, high-energy activities.
Water: Reactant and Product
Water (H2O) is not merely a solvent but an active participant in cellular metabolism. It serves as both a reactant and a product in various metabolic reactions.
Water molecules are involved in hydrolysis reactions, where larger molecules are broken down. Water is also a product of dehydration synthesis, where smaller molecules combine to form larger ones.
Carbon Dioxide: The Waste Product
Carbon dioxide (CO2) is a waste product generated during the breakdown of glucose and other molecules. It’s a testament to the complete oxidation of fuel sources within the cell.
Cells must efficiently eliminate carbon dioxide to maintain optimal pH levels and prevent toxic buildup. In multicellular organisms, the respiratory system facilitates this removal.
NAD+ and NADH: Electron Transfer Coenzymes
NAD+ (nicotinamide adenine dinucleotide) and its reduced form, NADH, are essential coenzymes that function as electron carriers.
NAD+ accepts electrons during metabolic reactions, becoming NADH. NADH then carries these electrons to the electron transport chain, where they are used to generate ATP.
The cyclical interconversion between NAD+ and NADH is crucial for redox reactions. These reactions drive energy production in the cell.
FAD and FADH2: Additional Electron Carriers
Similar to NAD+/NADH, FAD (flavin adenine dinucleotide) and its reduced form, FADH2, are also important electron carriers. They play a key role in the Krebs cycle and the electron transport chain.
FAD accepts electrons, forming FADH2, which then transports these electrons to the electron transport chain. FADH2 contributes to ATP production, though typically to a lesser extent than NADH.
Enzymes: Biological Catalysts
Enzymes are biological catalysts, typically proteins, that significantly accelerate the rate of metabolic reactions. They achieve this by lowering the activation energy required for reactions to occur.
Each enzyme is highly specific to a particular reaction or set of reactions. They are essential for maintaining the precise control and efficiency of metabolic pathways. Without enzymes, metabolic reactions would proceed too slowly to sustain life.
Life’s processes are deeply intertwined with the intricate world of cellular metabolism. Understanding this fundamental principle opens doors to comprehending how cells function and sustain life. Now that we’ve established a clear definition and grasp the significance of cellular metabolism, it’s time to dissect the core equation that governs this vital process.
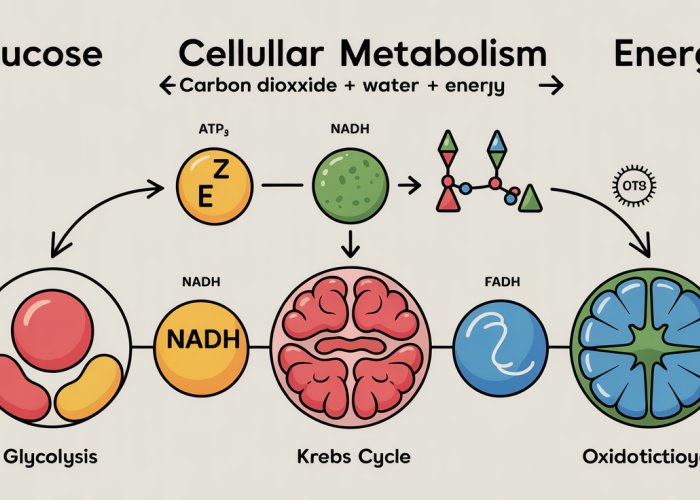
The Stages of Cellular Metabolism: A Step-by-Step Journey
Cellular metabolism isn’t a single event, but rather a carefully choreographed series of biochemical reactions. Each stage builds upon the previous one, extracting energy from fuel molecules and ultimately producing ATP, the cell’s energy currency. This section will explore the key stages of this journey: glycolysis, the intermediate step, the Krebs cycle, and the electron transport chain, examining their individual contributions to energy production.
Glycolysis: The Initial Breakdown of Glucose
Glycolysis, meaning "sugar splitting," marks the initial breakdown of glucose. This process occurs in the cytoplasm of the cell.
It’s a universal pathway found in nearly all organisms, highlighting its fundamental importance.
During glycolysis, a glucose molecule (a six-carbon sugar) is broken down into two molecules of pyruvate (a three-carbon molecule). This breakdown generates a small amount of ATP directly.
Reactants and Products of Glycolysis
The primary reactant in glycolysis is, of course, glucose. The main product is pyruvate.
However, the process also involves other key molecules, such as ATP (used in the early stages) and NADH (an electron carrier).
ATP Yield from Glycolysis
Glycolysis results in a net gain of 2 ATP molecules per glucose molecule. While this is a relatively small amount compared to later stages, it’s a crucial initial step in energy production. It also generates 2 molecules of NADH which will be used in the electron transport chain.
Intermediate Step: Pyruvate to Acetyl CoA
Before pyruvate can enter the Krebs cycle, it undergoes a crucial preparatory step.
In this step, pyruvate is transported into the mitochondria and converted into Acetyl Coenzyme A (Acetyl CoA).
This process releases carbon dioxide and generates NADH. Acetyl CoA then serves as the primary fuel for the Krebs cycle.
The Krebs Cycle (Citric Acid Cycle): Further Oxidation
The Krebs cycle, also known as the citric acid cycle, takes place in the mitochondria, the powerhouse of the cell. This cyclical pathway further oxidizes the Acetyl CoA derived from pyruvate.
Reactants and Products of the Krebs Cycle
Acetyl CoA combines with oxaloacetate to form citrate, which then undergoes a series of reactions. These reactions regenerate oxaloacetate and release carbon dioxide, ATP, NADH, and FADH2.
Generation of Electron Carriers
The Krebs cycle’s significance lies in its generation of electron carriers: NADH and FADH2. These molecules are crucial as they transport high-energy electrons to the electron transport chain. This is where the bulk of ATP production occurs.
Electron Transport Chain (ETC) and Oxidative Phosphorylation: The Major ATP Generator
The electron transport chain (ETC) is located in the inner mitochondrial membrane. It is the final stage of aerobic cellular respiration.
The Role of Oxygen
Oxygen serves as the final electron acceptor in the ETC. Electrons from NADH and FADH2 are passed along a series of protein complexes. Ultimately, they combine with oxygen and hydrogen ions to form water. This process releases energy, which is used to pump protons (H+) across the inner mitochondrial membrane, creating a proton gradient.
ATP Synthase and Chemiosmosis
The proton gradient established by the ETC drives ATP synthesis through a process called chemiosmosis. Protons flow back across the membrane through a protein complex called ATP synthase, using the energy of the gradient to convert ADP into ATP.
Significant ATP Production
The ETC is responsible for the vast majority of ATP produced during cellular respiration. Under ideal conditions, one glucose molecule can yield approximately 32-34 ATP molecules through this process.
Cellular Respiration: Aerobic vs Anaerobic
Cellular respiration is most efficient in the presence of oxygen (aerobic respiration). However, cells can also generate energy in the absence of oxygen (anaerobic respiration), though less efficiently.
When oxygen is limited, cells resort to processes like fermentation.
These pathways produce ATP without using oxygen, but they also generate different end products (such as lactic acid or ethanol) and yield significantly less ATP.
Life’s processes are deeply intertwined with the intricate world of cellular metabolism. Understanding this fundamental principle opens doors to comprehending how cells function and sustain life. Now that we’ve established a clear definition and grasp the significance of cellular metabolism, it’s time to dissect the core equation that governs this vital process.
Alternative Pathways: Life Without Oxygen
Cellular respiration, with its dependence on oxygen, is the primary energy-generating pathway in most organisms. But what happens when oxygen becomes scarce, or entirely absent? Cells have evolved ingenious alternative metabolic pathways to survive in these anaerobic conditions. These pathways, primarily fermentation, allow cells to continue generating ATP, albeit at a much lower efficiency.
Fermentation: An Anaerobic Solution
Fermentation is a metabolic process that converts sugars to acids, gases, or alcohol. It occurs in the absence of oxygen. While cellular respiration yields a substantial amount of ATP, fermentation produces only a fraction of that. This difference is due to the incomplete oxidation of glucose.
When and Why Fermentation Occurs
Fermentation kicks in when cells are deprived of oxygen. This can happen in several scenarios:
-
During intense exercise: Muscle cells may not receive enough oxygen to meet their energy demands, leading to lactic acid fermentation.
-
In microorganisms: Certain bacteria and yeast thrive in anaerobic environments and rely solely on fermentation for energy.
-
In specific tissues: Some tissues, like red blood cells, lack mitochondria and therefore rely on glycolysis and fermentation for their energy needs.
The primary purpose of fermentation is to regenerate NAD+, a crucial coenzyme required for glycolysis. Glycolysis itself produces a small amount of ATP, but it also consumes NAD+. Without a mechanism to replenish NAD+, glycolysis would quickly grind to a halt.
Fermentation steps in to convert pyruvate (the end product of glycolysis) into other compounds. This conversion regenerates NAD+, allowing glycolysis to continue and providing a limited, but essential, supply of ATP.
Types of Fermentation: Lactic Acid and Ethanol
There are several types of fermentation, but two of the most common are lactic acid fermentation and ethanol fermentation.
Lactic Acid Fermentation
In lactic acid fermentation, pyruvate is converted to lactic acid. This process occurs in muscle cells during strenuous activity, as well as in certain bacteria used to produce yogurt and cheese.
Ethanol Fermentation
In ethanol fermentation, pyruvate is converted to ethanol and carbon dioxide. This process is carried out by yeast and is used in the production of alcoholic beverages and bread.
Comparing ATP Yield: Fermentation vs. Cellular Respiration
The most striking difference between fermentation and cellular respiration is the ATP yield. Cellular respiration can generate up to 38 ATP molecules per glucose molecule. In contrast, fermentation produces only 2 ATP molecules per glucose molecule. This significant difference highlights the energetic advantage of aerobic metabolism.
To illustrate, consider these values:
-
Cellular Respiration: ~38 ATP per glucose molecule
-
Fermentation: 2 ATP per glucose molecule
The Role of Metabolic Pathways in Overall Metabolism
While fermentation is an alternative pathway activated under specific conditions, understanding its place within the larger metabolic network is crucial. It’s not merely an isolated event, but rather an interwoven component of cellular energy management.
The metabolic pathways, including glycolysis, the Krebs cycle, the electron transport chain, and fermentation, collaborate and compensate for each other based on cellular needs and environmental conditions. This adaptability highlights the remarkable resilience of living cells in maintaining their essential functions.
Life’s processes are deeply intertwined with the intricate world of cellular metabolism. Understanding this fundamental principle opens doors to comprehending how cells function and sustain life. Now that we’ve established a clear definition and grasp the significance of cellular metabolism, it’s time to dissect the core equation that governs this vital process. While understanding these processes is crucial, they only paint half the picture. The ebb and flow of energy within a cell is not just about breaking things down to generate power; it’s also about building new molecules and structures.
Building Up and Breaking Down: Anabolism and Catabolism
Cellular metabolism is a multifaceted process that encompasses two fundamental and interconnected pathways: anabolism and catabolism. These two processes work in concert to maintain cellular equilibrium, ensuring that cells have the resources they need to grow, repair, and perform their functions. Understanding the distinction between anabolism and catabolism is crucial for comprehending the overall dynamics of cellular metabolism.
Anabolism: The Building Phase
Anabolism refers to the set of metabolic processes that build complex molecules from simpler ones. These processes are endergonic, meaning they require an input of energy, typically in the form of ATP. Anabolism is essential for:
-
Growth and Development: Constructing new cells and tissues.
-
Repair: Replacing damaged cellular components.
-
Storage: Synthesizing macromolecules like glycogen and triglycerides for later use.
Key Anabolic Processes
Several key anabolic processes are critical for cellular function:
-
Protein Synthesis: Amino acids are linked together to form proteins, essential for structure, function, and regulation.
-
DNA Replication: Nucleotides are assembled to create new DNA strands, ensuring genetic information is passed on during cell division.
-
Polysaccharide Synthesis: Simple sugars like glucose are joined to form complex carbohydrates such as glycogen or starch.
-
Lipid Synthesis: Fatty acids and glycerol are combined to form lipids, including triglycerides for energy storage and phospholipids for cell membranes.
Catabolism: The Breakdown Phase
Catabolism, conversely, involves the breakdown of complex molecules into simpler ones. These processes are exergonic, meaning they release energy, often captured in the form of ATP. Catabolism is essential for:
-
Energy Production: Breaking down fuel molecules like glucose and fats to generate ATP.
-
Waste Removal: Degrading cellular waste products for excretion.
-
Recycling: Breaking down damaged or non-functional cellular components for reuse.
Key Catabolic Processes
Several catabolic processes are vital for energy production and cellular maintenance:
-
Glycolysis: Glucose is broken down into pyruvate, yielding a small amount of ATP and NADH.
-
Beta-Oxidation: Fatty acids are broken down into acetyl-CoA, generating NADH and FADH2.
-
Proteolysis: Proteins are broken down into amino acids, which can be used for energy or new protein synthesis.
-
The Citric Acid Cycle (Krebs Cycle): Acetyl-CoA is oxidized, generating ATP, NADH, FADH2, and carbon dioxide.
The Interplay of Anabolism and Catabolism
Anabolism and catabolism are not independent processes; they are tightly regulated and interconnected. The energy released from catabolic reactions is often used to drive anabolic reactions, creating a dynamic cycle of building up and breaking down. This delicate balance ensures that cells have the energy and building blocks they need to function optimally. The balance shifts based on cellular needs and environmental conditions.
For instance, after a meal, anabolic processes are favored as the body stores excess nutrients. During periods of fasting or exercise, catabolic processes dominate to provide energy. Understanding this intricate interplay is fundamental to comprehending the complexities of cellular metabolism and its role in maintaining life.
Building new molecules and breaking them down requires precise coordination. Without it, cells would either deplete their resources or accumulate toxic byproducts.
That’s where the sophisticated regulatory mechanisms of cellular metabolism come into play. These mechanisms ensure that metabolic pathways operate efficiently, responding dynamically to the cell’s ever-changing needs and environmental conditions.
Regulation: Keeping Metabolism in Check
Cellular metabolism is not a static process. It’s a highly dynamic and regulated network of biochemical reactions. This regulation ensures that cells can efficiently produce energy and synthesize essential molecules, adapting to fluctuations in nutrient availability, energy demands, and environmental conditions.
The cell employs a variety of sophisticated control mechanisms to maintain metabolic homeostasis, preventing wasteful energy expenditure and ensuring optimal resource utilization. These mechanisms include feedback inhibition, hormonal control, and enzyme regulation.
Feedback Mechanisms: Fine-Tuning Metabolic Pathways
Feedback mechanisms play a crucial role in maintaining metabolic balance. These mechanisms involve the products of metabolic pathways inhibiting earlier steps in the same pathway, preventing overproduction and conserving resources.
This type of regulation, known as feedback inhibition, is analogous to a thermostat controlling temperature in a house.
For example, in glycolysis, the accumulation of ATP can inhibit the enzyme phosphofructokinase, a key regulatory point in the pathway. This inhibition slows down the rate of glycolysis, preventing excessive ATP production when energy demands are low.
Similarly, the end products of amino acid synthesis pathways often inhibit the enzymes involved in their own production. This prevents the cell from wasting energy synthesizing amino acids that are already abundant.
Hormonal Control: Orchestrating System-Wide Metabolic Responses
Hormones act as chemical messengers, coordinating metabolic activities across different tissues and organs. They exert their effects by binding to specific receptors on cells, triggering signaling cascades that ultimately alter enzyme activity and gene expression.
Insulin, for example, is a key hormone involved in regulating glucose metabolism. When blood glucose levels are high, insulin is released, stimulating glucose uptake by cells and promoting glycogen synthesis in the liver and muscles. This helps to lower blood glucose levels and maintain glucose homeostasis.
In contrast, glucagon is released when blood glucose levels are low. It stimulates the breakdown of glycogen in the liver, releasing glucose into the bloodstream and increasing blood glucose levels.
Other hormones, such as epinephrine and cortisol, also play important roles in regulating metabolism during stress, exercise, and other physiological challenges.
The Role of the Pancreas
The pancreas, a vital organ in metabolic regulation, contains specialized cells called islets of Langerhans. These islets secrete insulin and glucagon in response to fluctuating glucose levels. This interplay between insulin and glucagon is essential for maintaining glucose homeostasis.
Enzyme Regulation: Controlling the Catalysts of Life
Enzymes, the biological catalysts that drive metabolic reactions, are themselves subject to various regulatory mechanisms. These mechanisms include:
-
Allosteric Regulation: Modulator molecules bind to enzymes, changing their shape and activity.
-
Covalent Modification: Chemical groups (e.g., phosphate) are added or removed, altering enzyme activity.
-
Enzyme Synthesis/Degradation: Cells control enzyme levels by regulating their production or breakdown.
Allosteric Regulation
Allosteric regulation involves the binding of modulator molecules to enzymes at sites distinct from the active site. This binding can either activate or inhibit enzyme activity, depending on the nature of the modulator and the enzyme.
For example, the enzyme hemoglobin, which carries oxygen in the blood, is allosterically regulated by oxygen itself. The binding of one oxygen molecule to hemoglobin increases the affinity of hemoglobin for subsequent oxygen molecules, a phenomenon known as cooperativity.
Covalent Modification
Covalent modification involves the addition or removal of chemical groups to enzymes, altering their activity. Phosphorylation, the addition of a phosphate group, is a common form of covalent modification that can either activate or inhibit enzymes.
Protein kinases are enzymes that catalyze the phosphorylation of other proteins, while protein phosphatases catalyze the removal of phosphate groups. The balance between kinase and phosphatase activity determines the phosphorylation state of enzymes and, consequently, their activity.
Enzyme Synthesis and Degradation
Cells can also regulate enzyme activity by controlling the rate at which enzymes are synthesized or degraded. This is a slower form of regulation compared to allosteric regulation or covalent modification, but it allows cells to adapt to long-term changes in metabolic demands.
For example, when glucose is abundant, cells increase the synthesis of enzymes involved in glycolysis and glycogen synthesis. Conversely, when glucose is scarce, cells decrease the synthesis of these enzymes and increase the synthesis of enzymes involved in gluconeogenesis (the synthesis of glucose from non-carbohydrate precursors).
Feedback mechanisms are essential for maintaining metabolic stability, preventing wasteful energy expenditure. Hormonal control also plays a key role, with hormones like insulin and glucagon orchestrating metabolic shifts in response to changes in blood glucose levels.
Enzymes, the catalysts of metabolic reactions, are themselves subject to regulation through various mechanisms such as allosteric control and covalent modification. But what other factors influence this incredibly intricate process?
Factors Influencing Metabolism: What Affects the Process?
Cellular metabolism, while governed by precise enzymatic reactions and regulatory mechanisms, is far from a fixed process. A multitude of factors can significantly impact its rate, efficiency, and even the specific pathways that are utilized. These factors range from the readily apparent, such as diet and exercise, to the more subtle influences of genetics and environmental exposures. Understanding these influences is crucial for comprehending how metabolism contributes to overall health and disease.
Environmental Factors and Metabolic Rate
The surrounding environment exerts a considerable influence on metabolic processes. Temperature, nutrient availability, and even exposure to toxins can all dramatically alter how cells generate energy and synthesize essential molecules.
Temperature and Metabolism
Temperature is a critical environmental factor affecting metabolic rate. Within reasonable physiological limits, an increase in temperature generally accelerates metabolic reactions.
This is because higher temperatures provide more kinetic energy, increasing the likelihood of successful collisions between enzymes and their substrates. However, extreme temperatures can denature enzymes, disrupting metabolic processes entirely.
Nutrient Availability and Metabolic Adaptation
The availability of nutrients, particularly glucose and other fuels, is a primary driver of metabolic activity. During periods of nutrient abundance, cells tend to favor anabolic pathways, storing energy and building complex molecules. Conversely, during periods of nutrient scarcity, catabolic pathways are activated to break down stored reserves and generate energy.
Toxins and Metabolic Disruption
Exposure to environmental toxins can severely disrupt metabolic processes. Many toxins interfere with enzyme function, block specific metabolic pathways, or damage cellular organelles involved in energy production.
For example, certain pesticides can inhibit the electron transport chain, impairing ATP synthesis and leading to cellular dysfunction.
Genetic Factors and Metabolic Predisposition
Genetic factors play a significant role in determining an individual’s metabolic profile. Variations in genes encoding metabolic enzymes, regulatory proteins, and nutrient transporters can influence metabolic rate, substrate utilization, and susceptibility to metabolic disorders.
Gene Variants and Enzyme Activity
Polymorphisms, or variations, in genes encoding metabolic enzymes can affect enzyme activity, substrate affinity, and regulatory properties. These variations can lead to differences in metabolic efficiency, drug metabolism, and nutrient utilization.
Genetic Predisposition to Metabolic Disorders
Certain genetic mutations can predispose individuals to metabolic disorders such as diabetes, obesity, and phenylketonuria (PKU). These mutations often disrupt key metabolic pathways, leading to the accumulation of toxic intermediates or the deficiency of essential metabolites.
Disease States and Metabolic Alterations
Disease states, both acute and chronic, can profoundly impact cellular metabolism. Infections, inflammation, and chronic diseases like cancer can alter metabolic pathways, energy demands, and nutrient requirements.
Infection and Metabolic Demand
Infections trigger an inflammatory response that increases metabolic demand. Immune cells require substantial energy and nutrients to combat pathogens, diverting resources from other tissues and altering systemic metabolism.
Cancer and Metabolic Reprogramming
Cancer cells exhibit a unique metabolic profile characterized by increased glucose uptake and glycolysis, even in the presence of oxygen. This phenomenon, known as the Warburg effect, supports rapid cell proliferation and tumor growth.
Diabetes and Metabolic Dysregulation
Diabetes mellitus is a metabolic disorder characterized by hyperglycemia and impaired insulin signaling. This leads to dysregulation of glucose metabolism, lipid metabolism, and protein metabolism, resulting in a range of complications.
Environmental factors, genetics, and disease states can each significantly alter the metabolic landscape within our bodies. This understanding naturally leads to a crucial question: How does this knowledge translate into practical applications that impact our daily lives and overall well-being?
Practical Applications: Metabolism in Real Life
Cellular metabolism, often perceived as an abstract biochemical process, has far-reaching implications in our lives. Understanding its intricacies allows us to address critical issues related to health, disease, and performance optimization. From tailoring diets to developing groundbreaking drugs, metabolic knowledge provides a powerful lens through which we can improve human well-being.
Metabolism and the Landscape of Health and Disease
Metabolic dysfunction is a central theme in a wide range of diseases. Conditions like diabetes, obesity, and even cancer are fundamentally linked to disruptions in normal metabolic pathways.
Understanding these disruptions is the first step toward developing effective treatments and preventative strategies.
Diabetes: A Paradigm of Metabolic Disorder
Diabetes, particularly type 2, exemplifies how impaired metabolic regulation can lead to chronic disease. Insulin resistance, a hallmark of type 2 diabetes, disrupts glucose metabolism, leading to elevated blood sugar levels.
By understanding the specific metabolic pathways affected by insulin resistance, researchers can develop targeted therapies to improve glucose control and prevent long-term complications.
Cancer: Reprogramming Metabolism for Survival
Cancer cells often exhibit altered metabolic profiles compared to normal cells. They frequently rely on aerobic glycolysis, also known as the Warburg effect, even when oxygen is plentiful. This metabolic shift provides cancer cells with the building blocks and energy needed for rapid growth and proliferation.
Targeting these unique metabolic vulnerabilities is a promising area of cancer research, offering the potential to selectively kill cancer cells while sparing healthy tissues.
Dietary and Exercise Implications of Metabolic Insight
The food we eat and the way we move directly impact our metabolic processes. A deeper understanding of metabolism allows us to make informed choices about diet and exercise to optimize our health and performance.
The Metabolic Impact of Macronutrients
Different macronutrients (carbohydrates, fats, and proteins) are metabolized through distinct pathways. Understanding these pathways is essential for designing effective dietary strategies.
For example, low-carbohydrate diets can shift the body’s primary fuel source from glucose to fat, leading to ketogenesis and potential weight loss. The effectiveness and safety of such diets depend heavily on individual metabolic profiles and overall health status.
Exercise and Metabolic Adaptation
Exercise is a powerful stimulus for metabolic adaptation. Endurance training, for instance, can increase mitochondrial density and improve the body’s ability to utilize fat as fuel.
Resistance training, on the other hand, can increase muscle mass, which in turn increases basal metabolic rate. Tailoring exercise regimens to individual metabolic goals requires a nuanced understanding of these adaptations.
Metabolism as a Target in Drug Development
Metabolic pathways are increasingly recognized as promising targets for drug development. By modulating specific metabolic enzymes or pathways, researchers can develop novel therapies for a wide range of diseases.
Targeting Metabolic Enzymes
Many drugs exert their effects by inhibiting or activating specific metabolic enzymes.
For example, statins, used to lower cholesterol, inhibit an enzyme involved in cholesterol synthesis. The development of new drugs that target metabolic enzymes requires a detailed understanding of enzyme kinetics and regulation.
Metabolic Reprogramming as Therapy
Some diseases, like cancer, involve significant reprogramming of cellular metabolism. Drugs that can reverse or counteract these metabolic shifts hold great therapeutic potential.
For instance, researchers are exploring the use of metformin, a commonly used diabetes drug, as a potential anti-cancer agent due to its effects on cellular metabolism.
Understanding the intricate interplay between metabolism, health, and disease is not just an academic exercise. It’s a key to unlocking new strategies for preventing and treating diseases, optimizing our diets and exercise regimens, and developing innovative therapies that target the very essence of cellular life.
So, there you have it – a comprehensive look at the cellular metabolism equation! Hopefully, this guide has made things a little clearer. Now go forth and apply your newfound knowledge!