Cellular life hinges on the intricate functionality of its fundamental building blocks. Phospholipids, a critical component of cell membranes, establish the bilayer structure that separates the cell’s internal environment. Understanding cell membrane macromolecules, including proteins and carbohydrates, is paramount for comprehending cellular processes. The National Institutes of Health (NIH) recognize the significance of this area, funding extensive research into the structure and function of these complex structures. Spectroscopy, a powerful analytical technique, provides crucial data for characterizing the composition and interactions of cell membrane macromolecules. Finally, the groundbreaking work of Singer and Nicolson’s fluid mosaic model continues to influence our understanding of the dynamic arrangement of these macromolecules within the cell membrane.
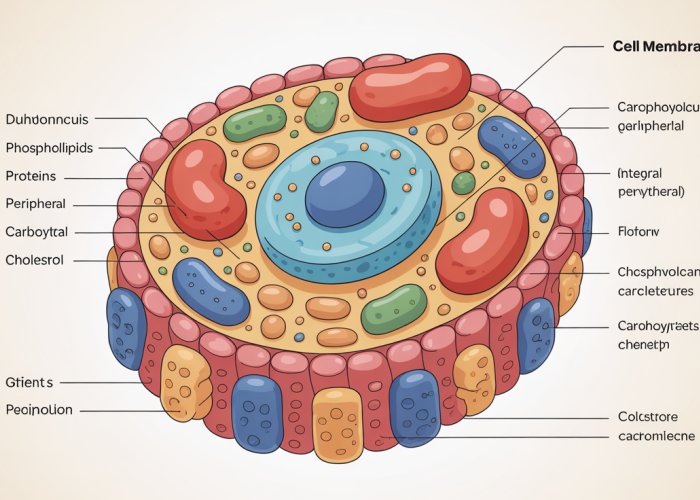
Unveiling the Secrets of the Cell Membrane
The cell membrane, a seemingly simple boundary, is in reality a highly sophisticated and dynamic structure. It’s the gatekeeper, the communicator, and the architect of cellular life, all rolled into one.
Its importance to life cannot be overstated.
This intricate barrier dictates what enters and exits the cell, maintains cellular integrity, and facilitates communication with the outside world. Without it, life as we know it would simply cease to exist.
The Vital Role of the Cell Membrane
Consider the cell membrane as the first line of defense and the central control unit of the cell. It’s responsible for maintaining the delicate internal environment necessary for all cellular processes to occur.
This includes regulating ion concentrations, nutrient uptake, and waste removal. It is also crucial for maintaining cell shape and volume.
Furthermore, the cell membrane plays a vital role in cell-to-cell communication and recognition, allowing cells to coordinate their activities and respond to external stimuli.
The Macromolecular Cast: Phospholipids, Proteins, Carbohydrates, and Cholesterol
The cell membrane isn’t a homogenous entity; it’s a complex assembly of diverse macromolecules, each playing a critical role in its structure and function. These key players include:
-
Phospholipids: Forming the structural foundation of the membrane, they create a barrier that separates the inside of the cell from the outside world.
-
Proteins: Acting as workhorses, they facilitate transport, signaling, and enzymatic reactions within the membrane.
-
Carbohydrates: Attached to lipids and proteins, they are involved in cell recognition and communication.
-
Cholesterol: Embedded within the lipid bilayer, it helps regulate membrane fluidity and stability.
Understanding the individual roles of each of these macromolecules and how they interact with each other is key to understanding the cell membrane’s overall function.
Purpose of Exploration: Decoding Macromolecular Roles
This article aims to dissect the intricate structure and function of the cell membrane by examining the roles of its constituent macromolecules. We will delve into the specific properties of phospholipids, proteins, carbohydrates, and cholesterol.
We aim to elucidate how each component contributes to the membrane’s overall architecture, dynamics, and functional capabilities.
By exploring these fundamental building blocks, we hope to provide a clear and comprehensive understanding of this vital cellular structure and its significance in maintaining life.
The cell membrane, teeming with diverse macromolecules, relies on a foundational element for its very structure. It’s time to delve into these primary building blocks of the cell membrane, the phospholipids, to fully appreciate the cell’s outer boundary.
Phospholipids: The Foundation of the Membrane
Phospholipids are the unsung heroes of the cell membrane, the very bedrock upon which its diverse functions are built. Their unique structure allows them to spontaneously assemble into a lipid bilayer, creating an effective barrier between the cell’s interior and the external environment. Without this carefully arranged architecture, life as we know it could not exist.
Amphipathic Nature of Phospholipids
The magic of phospholipids lies in their amphipathic nature, meaning they possess both hydrophilic (water-loving) and hydrophobic (water-fearing) regions.
Each phospholipid molecule consists of a polar head group, containing a phosphate group and an alcohol, which is attracted to water.
Attached to this head are two nonpolar fatty acid tails, which are repelled by water.
This dual nature is key to their ability to form bilayers.
The Lipid Bilayer: A Self-Assembling Barrier
In an aqueous environment, phospholipids spontaneously arrange themselves to minimize the exposure of their hydrophobic tails to water.
They do this by forming a lipid bilayer, where the hydrophilic heads face outwards, interacting with the surrounding water, and the hydrophobic tails cluster together in the interior, away from water.
This arrangement creates a stable barrier that is impermeable to many water-soluble molecules, effectively separating the cell’s internal environment from the external world.
This self-assembly process is driven by the hydrophobic effect and doesn’t require energy input.
It’s a testament to the elegant simplicity of nature’s designs.
Fluidity: A Dynamic and Essential Property
The lipid bilayer isn’t a static structure; it’s more like a dynamic, fluid sea.
Phospholipids are not rigidly fixed in place; they can move laterally within their own layer.
This fluidity is crucial for several reasons:
-
Membrane Flexibility: It allows the membrane to change shape, which is important for cell growth, division, and movement.
-
Protein Movement: It enables membrane proteins to diffuse laterally within the bilayer, allowing them to interact and carry out their functions.
-
Membrane Repair: It allows the membrane to self-seal if it is punctured or damaged.
The fluidity of the membrane is influenced by factors such as temperature and the composition of the fatty acid tails.
Saturated fatty acids, with their straight tails, pack together more tightly, decreasing fluidity.
Unsaturated fatty acids, with their kinks, prevent tight packing, increasing fluidity.
This balance is essential for maintaining proper membrane function.
The self-assembling nature of the lipid bilayer provides the structural foundation, but the cell membrane is far from a static, inert barrier. Embedded within and associated with this dynamic structure are proteins, the true workhorses of the membrane, carrying out a myriad of essential functions. It is through these proteins that the membrane truly comes alive, facilitating communication, transport, and enzymatic reactions.
Proteins: The Multifunctional Workhorses of the Membrane
Membrane proteins are the gatekeepers, messengers, and catalysts of the cell. They are responsible for most of the membrane’s specific functions, far beyond simply acting as a barrier.
From transporting nutrients and ions to receiving external signals and catalyzing reactions, proteins are essential for cellular life. These proteins can be broadly classified into two major categories based on their association with the lipid bilayer: integral and peripheral.
Integral Membrane Proteins: Embedded Within
Integral membrane proteins are permanently embedded within the lipid bilayer.
They possess hydrophobic regions that interact directly with the hydrophobic core of the bilayer, anchoring them firmly in place.
Transmembrane Proteins: A Key Subtype
Many integral proteins are also transmembrane proteins, meaning they span the entire width of the membrane.
These proteins have regions exposed to both the cell’s interior and exterior environments.
This allows them to act as channels or carriers, facilitating the transport of molecules across the membrane. The hydrophobic regions of transmembrane proteins typically consist of alpha-helices or beta-sheets with hydrophobic amino acid side chains.
These structures interact favorably with the lipid tails of the bilayer, ensuring stable integration.
Interaction with the Lipid Bilayer
The interaction between integral proteins and the lipid bilayer is crucial for maintaining membrane structure and function.
The hydrophobic regions of the protein are essential for anchoring it within the membrane, while the hydrophilic regions interact with the aqueous environments on either side.
Peripheral Membrane Proteins: Associated with the Surface
Peripheral membrane proteins, in contrast to integral proteins, do not embed themselves within the lipid bilayer.
Instead, they associate with the membrane indirectly, through interactions with integral membrane proteins or with the polar head groups of phospholipids.
Looser Association
This association is typically weaker than that of integral proteins, allowing peripheral proteins to be more easily detached from the membrane.
Peripheral proteins often play roles in cell signaling, enzyme activity, or maintaining the structural integrity of the membrane.
Interaction with the Lipid Bilayer
Peripheral proteins do not have distinct hydrophobic regions designed to insert into the lipid bilayer. Instead, they are anchored through interactions with the polar surfaces of the membrane.
These interactions can involve hydrogen bonds, ionic bonds, or other non-covalent interactions.
Diverse Functions of Membrane Proteins
The diverse array of membrane proteins enables the cell membrane to perform a wide range of functions, essential for cellular survival and communication.
These functions can be broadly categorized into transport, cell signaling, and enzymatic activity.
Transport: Gatekeepers of the Cell
Membrane proteins play a critical role in transporting molecules across the cell membrane.
This is essential for bringing in nutrients, removing waste products, and maintaining proper ion balance.
Channel proteins form pores or channels through the membrane, allowing specific molecules or ions to passively diffuse down their concentration gradients.
Carrier proteins bind to specific molecules and undergo conformational changes to shuttle them across the membrane.
These proteins can mediate both passive and active transport, depending on the molecule and the direction of transport.
Receptors for Cell Signaling: Receiving External Cues
Many membrane proteins act as receptors, binding to signaling molecules such as hormones, growth factors, or neurotransmitters.
This binding triggers a cascade of intracellular events, ultimately leading to a cellular response.
Membrane receptors are essential for cell-cell communication and for coordinating cellular activities in response to external stimuli.
Enzymes: Catalyzing Reactions
Some membrane proteins function as enzymes, catalyzing reactions that occur at the cell membrane.
These enzymes can be involved in a variety of processes, including lipid synthesis, signal transduction, and energy production.
Their strategic location within the membrane allows them to efficiently carry out these reactions and regulate cellular processes.
Carbohydrates: Cell Recognition and Communication
While lipids provide the structural framework and proteins execute diverse functions, carbohydrates on the cell membrane play a crucial, often overlooked, role in cellular identity and interaction. These sugar molecules, far from being mere decorations, are key players in cell recognition, adhesion, and signaling pathways. Their strategic placement on the cell surface allows for intricate communication between cells and their environment.
Glycoproteins and Glycolipids: Sugar-Coated Surfaces
Carbohydrates don’t exist in isolation on the cell membrane; they are covalently bonded to either lipids or proteins, forming glycolipids and glycoproteins, respectively. This attachment is typically on the extracellular side of the membrane, positioning the carbohydrate chains to interact with the external environment.
The specific sugars involved and their branching patterns are highly diverse, creating a unique "sugar code" on the cell surface. This diversity is critical for distinguishing between different cell types and mediating specific interactions.
Formation of Glycolipids
Glycolipids are formed when carbohydrates attach to lipid molecules within the cell membrane. The sugar moiety is linked to the polar head group of the lipid, extending outwards from the cell surface. These molecules are most abundant in nerve tissues.
Formation of Glycoproteins
Glycoproteins, on the other hand, involve the attachment of carbohydrates to proteins. This can occur at various amino acid residues on the protein, leading to a wide variety of glycosylation patterns. The process of glycosylation is complex, involving numerous enzymes and cellular machinery.
Cell-Cell Recognition and Adhesion: The Sugar Code in Action
The unique carbohydrate signatures on cell surfaces are essential for cell-cell recognition and adhesion. These interactions are vital for a multitude of biological processes, including:
-
Tissue Development: During embryonic development, cells use carbohydrate-mediated interactions to sort themselves and form organized tissues and organs.
-
Immune Responses: Immune cells recognize and target pathogens based on their surface carbohydrates. For example, blood type antigens are carbohydrate structures on red blood cells recognized by antibodies.
-
Inflammation: Carbohydrate-protein interactions mediate the adhesion of white blood cells to the site of inflammation, allowing them to migrate into the affected tissue.
This "sugar code" allows cells to distinguish "self" from "non-self," enabling targeted immune responses and preventing autoimmune reactions.
Carbohydrates in Cell Signaling Pathways
Beyond their role in direct cell-cell interactions, carbohydrates also participate in cell signaling pathways. Glycoproteins, in particular, can act as receptors for signaling molecules, triggering intracellular responses upon ligand binding.
Furthermore, modifications to cell surface carbohydrates can alter receptor activity and downstream signaling events. This provides an additional layer of regulation in cellular communication.
For example, certain growth factors bind to glycosylated receptors on cell surfaces, initiating signaling cascades that promote cell growth and proliferation. Aberrant glycosylation patterns have been implicated in various diseases, including cancer, highlighting the importance of carbohydrates in maintaining normal cellular function. The study of how cell surface carbohydrates affect cell signaling pathways is an ongoing field of research.
Glycoproteins and glycolipids contribute significantly to cellular identity and communication. However, another crucial component, cholesterol, plays a vital role in maintaining the membrane’s structural integrity and fluidity. Let’s delve into the unique properties and functions of cholesterol within the cell membrane.
Cholesterol: The Membrane’s Fluidity Regulator
Cholesterol, a sterol lipid, is a vital component of animal cell membranes. Its unique structure allows it to modulate membrane fluidity, ensuring optimal function across a range of temperatures.
Cholesterol’s Integration within the Lipid Bilayer
Cholesterol molecules are amphipathic, possessing both hydrophilic and hydrophobic regions.
This allows them to insert themselves into the lipid bilayer.
The hydroxyl (-OH) group, the polar region, interacts with the polar head groups of phospholipids.
The bulky steroid ring structure, the nonpolar region, associates with the hydrophobic fatty acid tails in the membrane’s interior.
This strategic positioning is crucial for cholesterol’s function.
The Impact of Cholesterol on Membrane Fluidity
High Temperatures: Reducing Fluidity
At high temperatures, the lipid bilayer becomes excessively fluid.
Cholesterol stabilizes the membrane by restricting the movement of phospholipid fatty acid tails.
The rigid steroid ring structure of cholesterol interacts with and limits the mobility of these tails.
This prevents the membrane from becoming too fluid and losing its structural integrity.
Low Temperatures: Preventing Solidification
At low temperatures, the lipid bilayer tends to become rigid and can even solidify.
Cholesterol prevents tight packing of phospholipid molecules.
Its presence disrupts the regular arrangement of fatty acid tails.
This lowers the temperature at which the membrane solidifies, maintaining fluidity even in cold conditions.
Maintaining Membrane Integrity: Cholesterol’s Crucial Role
Cholesterol acts as a buffer, preventing drastic changes in membrane fluidity due to temperature fluctuations.
This is essential for maintaining proper membrane function.
Enzymes, transport proteins, and receptor proteins within the membrane rely on optimal fluidity to function correctly.
Furthermore, maintaining membrane integrity prevents unwanted leakage of molecules across the membrane.
Without sufficient cholesterol, cells would be unable to adapt to changing environmental conditions.
This could compromise their ability to transport essential molecules and receive external signals.
The Fluid Mosaic Model: A Dynamic View of the Membrane
The cell membrane isn’t a static, rigid barrier. Instead, it’s a dynamic and ever-shifting structure. This understanding is encapsulated in the Fluid Mosaic Model, a concept that revolutionized our understanding of membrane biology.
This model portrays the cell membrane as a fluid lipid bilayer with proteins embedded within it, capable of lateral movement. It’s a far cry from earlier, more static conceptions.
Key Components of the Fluid Mosaic Model
At the heart of the Fluid Mosaic Model lies the understanding that the cell membrane is composed of several key components, each contributing to its unique properties:
-
The Phospholipid Bilayer: This forms the basic structure, providing a semi-permeable barrier. Phospholipids are arranged with their hydrophilic heads facing outwards. Their hydrophobic tails are facing inwards, creating a barrier to water-soluble substances.
-
Membrane Proteins: These are scattered throughout the lipid bilayer, like tiles in a mosaic. They perform a wide range of functions. Proteins can be integral (embedded within the bilayer) or peripheral (associated with the membrane surface).
-
Cholesterol: This sterol lipid is interspersed among the phospholipids. It helps to regulate membrane fluidity and stability.
-
Carbohydrates: These are attached to lipids (glycolipids) or proteins (glycoproteins) on the extracellular surface. They are involved in cell recognition and signaling.
The arrangement of these components is not fixed, hence the "fluid" nature of the model.
Lateral Movement: The Dance of Lipids and Proteins
A defining feature of the Fluid Mosaic Model is the emphasis on the lateral movement of lipids and proteins within the membrane.
-
Lipid Movement: Phospholipids can readily move laterally within their own monolayer. This movement contributes to the membrane’s fluidity. They can also rotate and flex their tails, further enhancing fluidity.
-
Protein Movement: Membrane proteins are also capable of lateral movement, although their movement can be more restricted than that of lipids. Some proteins are anchored to the cytoskeleton. Others are part of large complexes, limiting their mobility.
This lateral movement is not just a passive phenomenon. It’s crucial for many membrane functions. Receptor activation, signal transduction, and membrane trafficking all rely on the ability of membrane components to move and interact.
The Dynamic Nature of the Cell Membrane
The Fluid Mosaic Model highlights the ever-changing nature of the cell membrane. It’s not a static structure, but a dynamic assembly of lipids, proteins, and carbohydrates that are constantly in motion and interacting with each other.
-
Adaptation to Environmental Changes: The membrane can adjust its composition and fluidity in response to changes in temperature or other environmental factors. For example, cells may increase the proportion of unsaturated fatty acids in their phospholipids at low temperatures to maintain membrane fluidity.
-
Regulation of Cellular Processes: The dynamic nature of the membrane allows it to regulate a wide range of cellular processes. These include cell growth, cell division, and cell signaling.
-
Membrane Domains: Although the membrane is fluid, certain regions may be more organized than others, forming specialized domains with distinct functions. These domains can be enriched in specific lipids or proteins.
The Fluid Mosaic Model provides a powerful framework for understanding the complexity and versatility of the cell membrane. It emphasizes the importance of considering the membrane not as a simple barrier, but as a dynamic and interactive structure that plays a central role in cellular life.
With the Fluid Mosaic Model painting a picture of the membrane’s structure and dynamism, it’s natural to wonder how cells manage to shuttle essential molecules across this barrier. The selective traffic of molecules in and out of cells is orchestrated by sophisticated transport mechanisms, vital for maintaining cellular homeostasis and function.
Membrane Transport: Moving Molecules Across the Barrier
The cell membrane, while providing a barrier, is not impermeable. Cells must import nutrients, export waste, and maintain proper ion concentrations. This is accomplished through various membrane transport mechanisms. These mechanisms fall into two primary categories: passive transport, which requires no energy input from the cell, and active transport, which requires energy, typically in the form of ATP.
Passive Transport: Following the Gradient
Passive transport relies on the inherent kinetic energy of molecules and follows the laws of thermodynamics, specifically moving substances down their concentration gradients. This category includes diffusion, osmosis, and facilitated diffusion.
Diffusion: The Natural Flow
Diffusion is the simplest form of passive transport. It is the movement of molecules from an area of high concentration to an area of low concentration. This movement continues until equilibrium is reached. Small, nonpolar molecules like oxygen and carbon dioxide can readily diffuse across the lipid bilayer.
Osmosis: Water’s Journey
Osmosis is a special case of diffusion that focuses on the movement of water across a semipermeable membrane. Water moves from an area of high water concentration (low solute concentration) to an area of low water concentration (high solute concentration). This process is crucial for maintaining cell volume and preventing cell lysis or crenation.
Facilitated Diffusion: A Helping Hand
Facilitated diffusion also moves molecules down their concentration gradient. However, it requires the assistance of membrane proteins. These proteins, either channel or carrier proteins, provide a pathway for larger or polar molecules that cannot easily cross the lipid bilayer on their own. While it still follows the concentration gradient, the use of transport proteins speeds up this process.
Active Transport: Against the Odds
Active transport allows cells to move molecules against their concentration gradient. This is an energy-demanding process. It is typically fueled by ATP hydrolysis.
This mechanism is crucial for maintaining specific intracellular environments, like high potassium and low sodium concentrations, which are essential for nerve impulse transmission.
Primary active transport directly uses ATP to move molecules. A classic example is the sodium-potassium pump, which uses ATP to pump sodium ions out of the cell and potassium ions into the cell, both against their concentration gradients.
Secondary active transport harnesses the energy stored in an ionic gradient (created by primary active transport) to move other molecules. For example, the sodium-glucose cotransporter uses the sodium gradient to pull glucose into the cell, even if the glucose concentration is higher inside the cell than outside.
The Roles of Channels and Carrier Proteins
Both passive and active transport rely on specialized membrane proteins: channel proteins and carrier proteins.
Channel proteins form a pore through the membrane, allowing specific ions or small molecules to pass through. These channels can be gated, opening or closing in response to specific signals, such as changes in membrane potential or the binding of a ligand.
Carrier proteins bind to specific molecules and undergo a conformational change to transport the molecule across the membrane. These proteins are more selective than channel proteins and can be involved in both facilitated diffusion and active transport.
Understanding the nuances of membrane transport mechanisms is crucial for comprehending numerous biological processes, from nutrient uptake to nerve signaling. These processes are essential for cellular survival and function.
With the Fluid Mosaic Model painting a picture of the membrane’s structure and dynamism, it’s natural to wonder how cells manage to shuttle essential molecules across this barrier. The selective traffic of molecules in and out of cells is orchestrated by sophisticated transport mechanisms, vital for maintaining cellular homeostasis and function.
Selective Permeability: The Gatekeeper of the Cell
The cell membrane isn’t just a static barrier; it’s a highly regulated gateway. It embodies the principle of selective permeability, carefully controlling which substances can cross and which remain confined. This precise control is paramount for maintaining the cell’s internal environment, allowing it to thrive amidst fluctuating external conditions.
This section delves into the principles governing selective permeability, exploring the factors that determine a molecule’s ability to traverse the lipid bilayer. We’ll examine which molecules pass with ease and which require the assistance of specialized transport proteins, revealing the intricate mechanisms that underpin cellular life.
Understanding Selective Permeability
Selective permeability stems from the unique architecture of the cell membrane, primarily the phospholipid bilayer. The hydrophobic core of this bilayer presents a significant barrier to many molecules, particularly those that are charged or polar.
Imagine trying to mix oil and water – the same principle applies here. Hydrophobic molecules are compatible with the lipid interior, while hydrophilic (water-loving) molecules are repelled.
This inherent property dictates which substances can freely diffuse across the membrane and which require alternative routes.
The Easy Pass: Molecules That Diffuse Freely
Small, nonpolar molecules are the privileged few that can readily diffuse across the lipid bilayer. Oxygen ($O2$), carbon dioxide ($CO2$), and certain lipids belong to this category.
Their small size and lack of charge allow them to slip between the phospholipid molecules without significant resistance.
This passive movement, driven by concentration gradients, is essential for gas exchange, hormone signaling, and other fundamental cellular processes.
The Need for Assistance: When Transport Proteins Step In
Large, polar molecules and ions face a different challenge. Their size and charge prevent them from easily crossing the hydrophobic core of the membrane.
These molecules require the assistance of transport proteins, which act as gatekeepers, facilitating their passage across the membrane.
Glucose, amino acids, and ions like sodium ($Na^+$) and potassium ($K^+$) all rely on transport proteins to enter or exit the cell.
Types of Transport Proteins
Transport proteins come in two main flavors: channel proteins and carrier proteins.
-
Channel proteins form pores or tunnels through the membrane, allowing specific molecules or ions to flow through, often down their concentration gradients.
-
Carrier proteins, on the other hand, bind to specific molecules and undergo conformational changes to shuttle them across the membrane.
Both types of transport proteins play critical roles in regulating the flow of essential substances, ensuring that the cell receives the nutrients it needs and eliminates waste products effectively.
In essence, selective permeability is not just a property of the cell membrane; it’s a carefully orchestrated process that dictates the composition of the cellular environment. By controlling the movement of molecules in and out of the cell, the membrane maintains homeostasis and enables the complex functions that define life.
With the Fluid Mosaic Model painting a picture of the membrane’s structure and dynamism, it’s natural to wonder how cells manage to shuttle essential molecules across this barrier. The selective traffic of molecules in and out of cells is orchestrated by sophisticated transport mechanisms, vital for maintaining cellular homeostasis and function.
Cell Signaling: Receiving and Responding to External Cues
The cell membrane is far more than just a physical barrier; it’s also a crucial communication hub. Cells are constantly bombarded with external signals, ranging from hormones and growth factors to neurotransmitters and even physical stimuli like light or touch.
These signals dictate a wide range of cellular behaviors, including growth, differentiation, movement, and even programmed cell death (apoptosis). The cell membrane acts as the cell’s antenna, equipped with specialized receptors that detect and interpret these external cues.
These receptors then trigger a cascade of intracellular events, ultimately leading to a specific cellular response. This intricate process, known as cell signaling, is fundamental to multicellular life, allowing cells to coordinate their activities and maintain tissue homeostasis.
The Role of Membrane Receptors
Membrane receptors are proteins embedded within the cell membrane that act as the primary interface between the cell and its external environment. These receptors exhibit remarkable specificity, binding only to particular signaling molecules, also known as ligands.
This lock-and-key interaction ensures that cells respond appropriately only to the signals intended for them. Receptors can be broadly categorized based on their structure and mechanism of action.
Types of Membrane Receptors
- G protein-coupled receptors (GPCRs): These receptors are the largest family of membrane receptors and are involved in a vast array of cellular processes. Upon ligand binding, GPCRs activate intracellular G proteins, which then modulate the activity of other downstream effector proteins.
- Receptor tyrosine kinases (RTKs): RTKs are transmembrane receptors with intrinsic enzymatic activity. Ligand binding to RTKs triggers receptor dimerization and autophosphorylation, which in turn activates various intracellular signaling pathways.
- Ligand-gated ion channels: These receptors directly control the flow of ions across the cell membrane. Ligand binding opens the ion channel, allowing specific ions to flow down their electrochemical gradients.
- Enzyme-linked receptors: These receptors have an intracellular domain that is associated with an enzyme. Ligand binding activates the enzyme, initiating a signaling cascade.
Initiating Intracellular Signaling Cascades
The binding of a signaling molecule to its receptor is merely the first step in a complex signaling pathway. This initial event triggers a chain reaction of intracellular events, often involving a series of protein-protein interactions and post-translational modifications.
These signaling cascades amplify the original signal and relay it to various downstream effector proteins, ultimately leading to a change in cellular behavior.
Common Signaling Pathways
- The cAMP pathway: Activation of GPCRs can lead to the production of cyclic AMP (cAMP), a second messenger that activates protein kinase A (PKA). PKA then phosphorylates various target proteins, leading to diverse cellular responses.
- The MAPK pathway: Receptor tyrosine kinases often activate the mitogen-activated protein kinase (MAPK) pathway, which plays a crucial role in cell growth, proliferation, and differentiation.
- The calcium signaling pathway: Many signaling pathways involve changes in intracellular calcium levels. Calcium ions act as second messengers, binding to various proteins and triggering a wide range of cellular responses.
- The inositol phospholipid pathway: Activation of certain receptors can lead to the production of inositol phospholipids, which then activate protein kinase C (PKC) and other downstream effectors.
Examples of Cell Signaling Pathways
Cell signaling pathways are involved in virtually every aspect of cellular life. Here are a few examples of how they play a crucial role in various biological processes:
- Insulin signaling: Insulin binds to its receptor tyrosine kinase, triggering a signaling cascade that promotes glucose uptake and storage.
- Epinephrine signaling: Epinephrine (adrenaline) binds to GPCRs, leading to the activation of the cAMP pathway and a subsequent increase in heart rate and blood pressure.
- Nerve Growth Factor (NGF) signaling: NGF binds to its receptor, activating signaling pathways that promote the survival and differentiation of neurons.
- Wnt signaling: Wnt proteins bind to Frizzled receptors, activating intracellular signaling cascades that control cell fate and development.
These are just a few examples of the many cell signaling pathways that operate within our bodies. Understanding these pathways is crucial for developing new therapies for a wide range of diseases, including cancer, diabetes, and neurological disorders.
Frequently Asked Questions About Cell Membrane Macromolecules
Here are some common questions about cell membrane macromolecules and their crucial roles in cellular life.
What exactly are cell membrane macromolecules?
Cell membrane macromolecules are large biological molecules that form the structure and enable the function of the cell membrane. These primarily include lipids (like phospholipids and cholesterol), proteins, and carbohydrates. These components work together to create a flexible and selectively permeable barrier.
Why are cell membrane macromolecules so important?
Cell membrane macromolecules are critical because they control what enters and exits the cell, maintaining the internal environment. They also facilitate cell communication, provide structural support, and enable various essential cellular processes. The proper function of these molecules is vital for cell survival.
How do proteins function within the cell membrane?
Proteins embedded in the cell membrane perform diverse tasks. Some act as channels or carriers to transport specific molecules across the membrane. Others are receptors that bind to signaling molecules, triggering cellular responses. These protein functionalities contribute significantly to the cell’s ability to interact with its environment.
What happens if cell membrane macromolecules are damaged or dysfunctional?
Damage or dysfunction of cell membrane macromolecules can disrupt cell signaling, nutrient transport, and waste removal. This can lead to various cellular dysfunctions and diseases. Maintaining the integrity of these molecules is essential for overall cell health and proper function.
So, that’s the lowdown on cell membrane macromolecules! Hopefully, you now have a better grasp of how important they are for, well, everything. Go forth and spread the knowledge (or at least impress someone at your next trivia night!). Until next time!