Understanding atomic structure is crucial, and the Bohr model provides a simplified, yet powerful, visualization. Calcium (Ca), a vital element in biological systems and geological formations, possesses a unique electron configuration that’s often represented using the ca bohr diagram. Electron arrangement, an important factor in understanding chemical properties, is effectively described in this diagram. This article seeks to explain the ca bohr diagram using these concepts in under five minutes.
Decoding Calcium’s Atomic Structure: A 5-Minute Guide
Calcium. It’s more than just a mineral in your milk. It’s a fundamental element, a cornerstone of both the natural world and human biology.
From the strong bones that support our bodies to the complex signaling pathways within our cells, calcium plays a vital role. But what exactly is calcium, and how does its atomic structure dictate its behavior?
Introducing Calcium: The Element of Life
Calcium (Ca), with its familiar place on the periodic table, is an alkali earth metal. It’s abundant in the Earth’s crust and essential for life as we know it.
We encounter it daily, not just in dairy products but also in leafy green vegetables, supplements, and even certain medications. Its presence extends far beyond our dinner plates, however. Calcium compounds are found in rocks, soil, and even the vast oceans.
In the human body, calcium’s primary function is building and maintaining strong bones and teeth. But it also plays a crucial role in:
- Muscle function
- Nerve transmission
- Blood clotting
- Enzyme activity
Calcium deficiencies can lead to serious health problems, highlighting just how critical this element is.
What is a Bohr Diagram? Visualizing the Atom
To understand calcium’s unique properties, we need to delve into its atomic structure. That’s where the Bohr diagram comes in.
The Bohr diagram is a simplified representation of an atom. It illustrates the arrangement of electrons within different energy levels, or shells, around the nucleus.
Think of it as a roadmap of an atom, showing where the electrons, the negatively charged particles, are located. It’s a visual tool that helps us understand how atoms interact with each other and form chemical bonds.
The Bohr diagram is named after Niels Bohr, a Danish physicist who made groundbreaking contributions to our understanding of atomic structure. His model, while not entirely accurate by today’s standards, provides a valuable foundation for visualizing the atom.
Why Calcium’s Bohr Diagram Matters
Learning about Calcium’s Bohr Diagram provides insight into the behavior of this essential element. It allows us to:
- Understand its reactivity
- Predict how it will interact with other elements
- Appreciate its role in forming various compounds
By visualizing the arrangement of electrons in Calcium, we can better understand why it behaves the way it does. This knowledge is essential in fields ranging from medicine to materials science.
Our Goal: A Quick & Clear Explanation
In the next few minutes, we’ll break down the process of creating Calcium’s Bohr diagram step by step. We’ll focus on clarity and conciseness, ensuring that you grasp the key concepts without getting bogged down in complex details.
By the end of this guide, you’ll have a solid understanding of Calcium’s atomic structure and the power of the Bohr diagram.
Atomic Fundamentals: A Quick Recap
Before we jump into drawing Calcium’s Bohr diagram, let’s quickly revisit some fundamental concepts about atomic structure. This will provide a solid foundation for understanding how electrons are arranged around the Calcium atom’s nucleus, and why the Bohr diagram is such a useful tool.
Decoding Atomic Structure: Protons, Neutrons, and Electrons
Atoms are the basic building blocks of all matter. Each atom consists of a central nucleus surrounded by orbiting electrons.
The nucleus contains two types of particles: protons and neutrons. Protons carry a positive charge, while neutrons are electrically neutral.
Electrons, on the other hand, carry a negative charge and orbit the nucleus in specific energy levels or shells. The number of protons in an atom’s nucleus defines what element it is.
Understanding Electron Configuration: Organizing the Orbitals
Electron configuration describes the arrangement of electrons within an atom’s energy levels or shells. Electrons don’t just float randomly around the nucleus.
Instead, they occupy specific orbitals within these shells, following certain rules. The innermost shell can hold a maximum of two electrons, while the subsequent shells can hold more, as determined by the 2n2 rule, where ‘n’ is the shell number. This orderly arrangement dictates how an atom interacts with other atoms.
Calcium’s Atomic Number: The Key to its Identity
The atomic number is a fundamental property of an element. It represents the number of protons found in the nucleus of an atom of that element. Calcium (Ca) has an atomic number of 20.
This means that every Calcium atom possesses 20 protons within its nucleus. In a neutral atom, the number of protons is equal to the number of electrons. Therefore, a neutral Calcium atom also has 20 electrons orbiting its nucleus. This balance of charge is crucial for the atom’s stability.
Niels Bohr and the Atomic Model
Niels Bohr, a Danish physicist, made significant contributions to our understanding of atomic structure. His model, proposed in 1913, introduced the concept of quantized energy levels for electrons.
Bohr proposed that electrons orbit the nucleus in specific paths, or shells, with fixed energy levels. When an electron jumps from one energy level to another, it either emits or absorbs energy in the form of photons.
This revolutionary idea laid the groundwork for the development of the Bohr diagram, a simplified visual representation of the atom, which helps us understand how electrons are arranged and how they contribute to an element’s chemical properties. The Bohr model, while simplified, was a crucial stepping stone in the development of more complex and accurate atomic models.
The atomic number of an element is intrinsically linked to how its electrons are arranged. These electrons, governed by specific rules and principles, dictate the behavior of the element. Understanding electron arrangement helps to visually represent and predict the properties of each element. With the foundations established, we can now put theory into practice. Let’s now delve into a step-by-step guide to constructing Calcium’s Bohr diagram, visually mapping out its atomic structure.
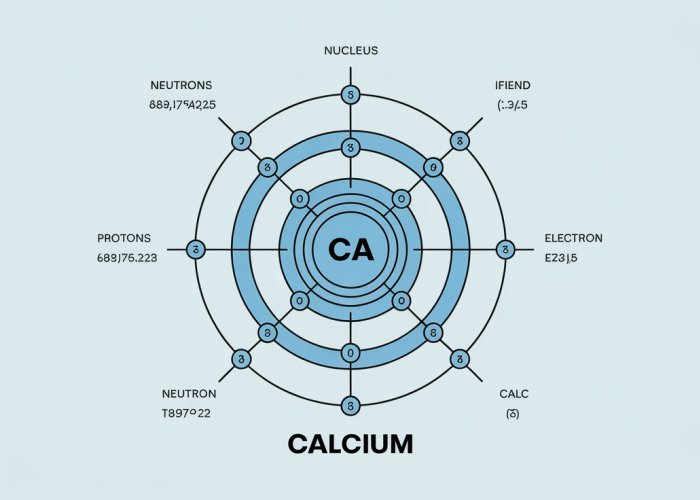
Drawing the Diagram: A Step-by-Step Guide
This section provides a practical, hands-on guide to creating a Bohr diagram for Calcium (Ca).
We’ll break down the process into manageable steps.
Each step is crucial to accurately represent Calcium’s atomic structure.
Determining the Number of Electron Shells
Calcium’s electrons are arranged into electron shells, otherwise known as energy levels.
The period number of an element in the periodic table indicates the number of electron shells in that element’s atoms.
Calcium is located in the fourth period of the periodic table.
Therefore, a Calcium atom possesses four electron shells.
Filling the Electron Shells: The 2, 8, 8, 2 Configuration
Electron configuration dictates how electrons populate these shells.
Calcium’s electron configuration is 2, 8, 8, 2.
This means that the first shell holds two electrons.
The second shell holds eight electrons.
The third shell also holds eight electrons.
And the outermost fourth shell has two electrons.
Understanding the 2n2 Rule
The 2n2 rule defines the maximum electron capacity in a given shell.
Here, ‘n’ represents the shell number.
The first shell (n=1) can hold 2(1)2 = 2 electrons.
The second shell (n=2) can hold 2(2)2 = 8 electrons.
The third shell (n=3) can hold 2(3)2 = 18 electrons.
Although the third shell can hold up to 18 electrons, elements like Calcium often fill it with 8 before occupying the next shell.
The fourth shell (n=4) can hold 2(4)2 = 32 electrons.
Identifying Valence Electrons
Valence electrons are the electrons residing in the outermost shell of an atom.
These electrons determine the element’s chemical properties.
Calcium has two valence electrons, residing in its outermost (fourth) shell.
Constructing the Bohr Diagram: A Visual Guide
Follow these steps to draw Calcium’s Bohr diagram:
-
Draw a circle in the center to represent the nucleus.
Write "Ca" inside to denote Calcium.
Also, include the number of protons (+20) and neutrons (typically 20, assuming the most common isotope Calcium-40) within the circle. -
Draw the first electron shell around the nucleus.
Place two dots (or crosses) on this shell to represent the two electrons in the first energy level. -
Draw the second electron shell around the first.
Place eight dots on this shell. -
Draw the third electron shell around the second.
Place eight dots on this shell. -
Finally, draw the fourth electron shell around the third.
Place two dots on this shell to represent the two valence electrons.
This completes the Bohr diagram for Calcium.
The diagram visually represents Calcium’s atomic structure.
It shows the arrangement of its electrons in their respective energy levels.
The principles governing electron configuration and Bohr diagrams are more than just abstract concepts. They provide a tangible link to the observable chemical behavior of elements. Let’s explore how the Bohr diagram of Calcium, meticulously constructed, reveals critical insights into its reactivity and role in forming chemical compounds.
Calcium’s Reactivity: Insights from the Bohr Diagram
The true power of the Bohr diagram lies in its ability to illuminate an element’s chemical properties. By visually representing electron arrangement, the diagram offers clues about how an element will interact with others. In Calcium’s case, understanding the Bohr diagram unlocks key insights into its reactivity and its propensity to form specific types of chemical bonds.
Valence Electrons: The Key to Reactivity
Valence electrons, those residing in the outermost shell, are the primary drivers of an atom’s chemical behavior. These electrons are the ones involved in forming chemical bonds with other atoms. Elements strive to achieve a stable electron configuration, typically resembling that of a noble gas, which has a full outer shell.
Calcium, as depicted in its Bohr diagram, possesses two valence electrons in its fourth and outermost shell. This is a crucial factor in determining its reactivity. Having only two valence electrons means Calcium does not have a stable electron configuration. Therefore, it is more likely to interact with other atoms in order to achieve stability.
The Formation of Ca2+ Ions
To attain a more stable electron configuration, Calcium readily loses its two valence electrons. By shedding these two electrons, Calcium achieves the same electron configuration as Argon (Ar), the noble gas preceding it in the periodic table.
When Calcium loses two negatively charged electrons, it becomes a positively charged ion with a 2+ charge (Ca2+). This process is known as oxidation, and it is a hallmark of Calcium’s chemical behavior.
The resulting Ca2+ ion has a stable electron configuration. Consequently, it is far less reactive than the neutral Calcium atom. The Bohr diagram effectively illustrates this transformation, showing the change in electron count and the resulting ionic charge.
Calcium as a Reactive Metal: Connecting Diagram to Properties
The Bohr diagram reveals why Calcium is categorized as a reactive metal. Metals, in general, tend to lose electrons to form positive ions. Calcium’s tendency to readily lose its two valence electrons aligns perfectly with this behavior.
This characteristic explains why Calcium is rarely found in its pure, elemental form in nature. Instead, it exists primarily as compounds. For example, as Calcium Carbonate (CaCO3) in limestone, or as Calcium Sulfate (CaSO4) in gypsum. The Bohr diagram makes it clear that Calcium’s reactivity drives its participation in forming these and other compounds.
Calcium’s Role in Compounds: A Consequence of Electron Configuration
The formation of Ca2+ ions directly influences the types of compounds Calcium forms. Positively charged Calcium ions are attracted to negatively charged ions, such as chloride (Cl–) or oxide (O2-).
This electrostatic attraction leads to the formation of ionic compounds. These are compounds where electrons are transferred between atoms rather than shared. Familiar examples include Calcium Chloride (CaCl2), used as a road salt, and Calcium Oxide (CaO), also known as quicklime, utilized in cement production.
The Bohr diagram, therefore, provides a visual rationale for understanding why Calcium combines with specific elements in particular ratios to form stable compounds. It illustrates the driving force behind ionic bond formation and highlights the predictive power of the Bohr model.
FAQs: Understanding Calcium’s Bohr Diagram
Here are some frequently asked questions to help you better understand the Bohr diagram of Calcium.
What does the Calcium Bohr diagram represent?
The calcium bohr diagram visually represents the arrangement of electrons in the different energy levels, or electron shells, surrounding the nucleus of a calcium atom. It shows how 20 electrons are distributed.
How many electrons are in Calcium’s outermost shell?
Calcium (Ca) has two electrons in its outermost shell. This is crucial because these valence electrons are responsible for calcium’s chemical reactivity and how it forms bonds with other elements.
Why is the Calcium Bohr diagram important?
The ca bohr diagram helps us understand calcium’s electronic configuration and therefore its chemical properties. It provides a simple, visual way to see how electrons are arranged, which is key to understanding its reactivity.
How does the Calcium Bohr diagram relate to Calcium’s reactivity?
The two electrons in Calcium’s outermost shell mean it readily loses these two electrons to achieve a stable, filled outer shell configuration. This tendency to lose electrons makes Calcium a reactive metal, and the ca bohr diagram shows us why.
So, there you have it! Hopefully, you now have a clearer picture of the ca bohr diagram. It’s a cool little illustration that really helps visualize how calcium’s electrons are arranged. Keep exploring and happy learning!