Argon, a noble gas identified by its symbol ‘Ar’ on the Periodic Table, possesses the noteworthy characteristic known as atomic number argon. This numerical identifier, specifically the number 18, defines argon’s position and properties within the framework of Chemistry. Dr. Eleanor Vance, a pioneering researcher in noble gas applications, highlights the significance of understanding atomic structure in predicting the behavior of elements like argon. Its inert nature makes it invaluable in applications ranging from welding, where it prevents oxidation, to lighting, where its presence prolongs filament life. Therefore, exploring atomic number argon unlocks the understanding of argon’s fundamental properties and its surprisingly diverse applications.
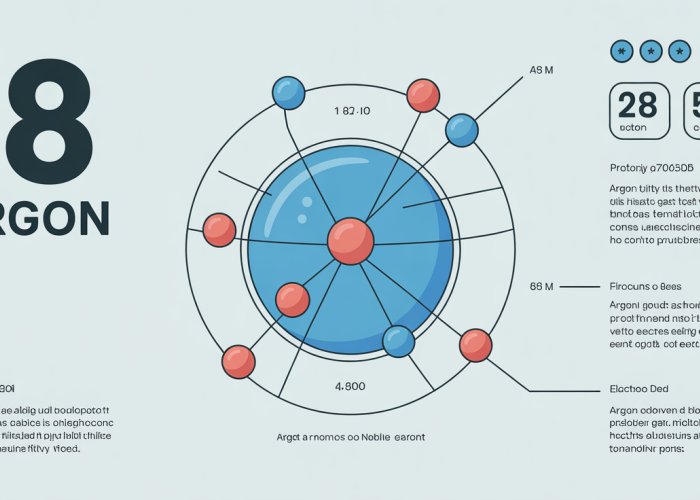
Argon (Ar), a colorless and odorless gas, often lurks in the background of our everyday lives, quietly contributing to a range of applications from welding to lighting. As a member of the noble gas family, Argon possesses unique characteristics that stem directly from its fundamental atomic structure.
At the heart of understanding Argon lies its atomic number, a seemingly simple integer that unlocks a wealth of information about this element’s behavior, properties, and interactions.
Grasping the significance of Argon’s atomic number is paramount to appreciating its role in various scientific and industrial contexts. Furthermore, it provides a gateway to understanding the broader principles of atomic structure and the organization of the periodic table.
Think of it this way: without the key of the atomic number, understanding the specific traits of Argon becomes a struggle.
The Atomic Number: A Fingerprint of the Elements
The atomic number is not just a random label; it’s a fundamental identifier, representing the number of protons found within the nucleus of an atom. This number is unique to each element and serves as its atomic fingerprint, distinguishing it from all others.
Therefore, the number of protons defines what element the atom truly is.
Argon’s Defining Number: 18
So, what number defines Argon? Argon’s atomic number is 18. This means that every Argon atom contains precisely 18 protons in its nucleus. This seemingly small detail dictates much of Argon’s observed behavior, from its chemical inertness to its spectral properties.
The atom can then be modeled and understood with this single piece of information.
Argon and the Periodic Table
The periodic table is an organized arrangement of the elements based on their atomic number and recurring chemical properties. Argon’s position on the periodic table, specifically within Group 18 (the noble gases), is directly determined by its atomic number.
This placement reflects its stable electron configuration and its characteristic lack of reactivity. Therefore, the atomic number can be seen as a guide when using the periodic table.
Argon’s unique atomic fingerprint leads us to a more fundamental question: what exactly is an atomic number, and why is it so important? To truly appreciate Argon’s place in the universe, we need to delve into the core concepts of atomic structure.
The Foundation: What is Atomic Number?
The atomic number isn’t just a label assigned to an element; it’s the very cornerstone of its identity. It’s the key to understanding its behavior, its properties, and its place within the grand scheme of the periodic table. Let’s break down this crucial concept.
Defining the Atomic Number: The Proton Count
The atomic number is defined as the number of protons found in the nucleus of an atom. This number is always a whole number and is absolutely unique to each element.
Think of it as an element’s social security number – no two elements share the same atomic number.
For example, every atom with one proton is, by definition, hydrogen. Every atom with two protons is helium, and so on. Change the number of protons, and you change the element itself.
The Unique Identifier of an Element
This simple count of protons acts as the definitive identifier for each element. It’s the fundamental property that distinguishes one element from another.
The atomic number dictates the element’s chemical behavior and its interactions with other atoms.
Without the atomic number, we would have no way of organizing or understanding the elements that make up the world around us. The organization of elements that we know of starts with the atomic number.
The Subatomic Trio: Protons, Neutrons, and Electrons
To fully grasp the significance of the atomic number, we must briefly touch upon the three primary subatomic particles that constitute an atom:
- Protons: Positively charged particles located in the nucleus. The number of protons determines the element’s atomic number.
- Neutrons: Neutral (no charge) particles also located in the nucleus. Neutrons contribute to the atom’s mass but do not affect its atomic number.
- Electrons: Negatively charged particles that orbit the nucleus in specific energy levels or shells. Electrons are responsible for chemical bonding and interactions.
The Charge Balance: Neutral Atoms
In a neutral atom (an atom with no overall electrical charge), the number of negatively charged electrons is exactly equal to the number of positively charged protons.
This balance of charges ensures that the atom as a whole is electrically neutral.
While the number of neutrons can vary (leading to isotopes, which we’ll discuss later), the number of electrons in a neutral atom is always dictated by the atomic number (the number of protons).
The organization of elements that we use today wouldn’t be possible without understanding the atomic number, it acts as a map to chart the properties of each element and understanding where they fit in the known world. This map, the periodic table, offers invaluable insights into how elements behave and interact.
Argon’s Place in the Periodic Table: The Noble Gas Family
The periodic table is more than just a list of elements; it’s a carefully organized chart that reflects recurring patterns in their properties. Where an element sits on this table tells us a great deal about its characteristics, and Argon is no exception.
Group 18: The Realm of the Noble Gases
Argon resides in Group 18, also known as the noble gases or inert gases.
This group sits on the far right-hand side of the periodic table, a position it earns due to its unique electronic structure. The noble gases—Helium, Neon, Argon, Krypton, Xenon, and Radon—are characterized by their exceptional stability and lack of reactivity.
The Octet Rule: The Key to Noble Gas Stability
The octet rule is central to understanding the stability of noble gases.
This rule states that atoms tend to gain, lose, or share electrons to achieve a full outer electron shell containing eight electrons (except for Helium, which aims for two, mimicking the electron configuration of Hydrogen).
Noble gases already possess this full outer shell, giving them an electron configuration that is exceptionally stable. Argon, with its electron configuration of 1s² 2s² 2p⁶ 3s² 3p⁶, has a complete outer shell. This complete configuration means it has little to no drive to form chemical bonds with other elements.
Inertness: Argon’s Defining Trait
Due to its stable electron configuration, Argon is generally considered inert, meaning it is chemically unreactive.
For many years, noble gases were thought to be completely incapable of forming compounds. However, under extreme conditions in laboratory settings, scientists have been able to coax Argon into forming compounds with highly electronegative elements like fluorine.
These compounds are rare and require very specific conditions to form and exist, but they demonstrate that even the most inert elements have their limits.
Pushing the Boundaries: Argon Compounds
While Argon is overwhelmingly unreactive, research has shown that it can form compounds.
Argon fluorohydride (HArF) is one such example, created by trapping Argon, hydrogen, and fluorine in a low-temperature matrix and then irradiating it with UV light. These compounds are extremely unstable and decompose quickly when the extreme conditions are removed.
The existence of Argon compounds, however fleeting, challenges our traditional understanding of chemical inertness and expands the scope of chemical possibilities. They highlight the fact that even the most fundamental rules in chemistry can be bent under extreme conditions, paving the way for new discoveries and a deeper understanding of how elements interact.
The dance of electrons around an atom’s nucleus dictates its chemical personality, and for Argon, this dance is a picture of serene stability. Having established Argon’s place as a noble gas and understood the octet rule, we can now delve deeper into the specifics of its electron configuration to fully appreciate its inert nature.
Electron Configuration of Argon: Defining its Inert Nature
Unpacking Argon’s Electronic Structure
Argon’s electron configuration is written as 1s² 2s² 2p⁶ 3s² 3p⁶.
This notation describes how Argon’s 18 electrons are arranged within its various electron shells and subshells.
Let’s break down what each part of this configuration signifies:
-
The numbers (1, 2, 3) represent the electron shells or energy levels, with 1 being closest to the nucleus and having the lowest energy.
-
The letters (s, p) denote the subshells or orbitals within each shell, each with a slightly different shape and energy.
-
The superscripts (², ⁶) indicate the number of electrons occupying each subshell.
This orderly arrangement is not arbitrary.
It follows specific rules that govern how electrons fill available energy levels, always striving for the lowest energy state possible.
The Significance of Full Electron Shells
The key to Argon’s inertness lies in its completely filled outer electron shell.
The outermost shell, also known as the valence shell, is where an atom interacts with other atoms.
For Argon, this is the third shell (n=3), which contains the 3s and 3p subshells.
Notice that the 3s subshell is filled with 2 electrons (3s²) and the 3p subshell is filled with 6 electrons (3p⁶).
This gives Argon a total of eight electrons in its valence shell, fulfilling the octet rule.
This stable octet configuration means Argon has little to no tendency to gain, lose, or share electrons, making it exceptionally unreactive under normal circumstances.
A Brief Look at Atomic Orbitals
To further understand electron configuration, a brief discussion of atomic orbitals is warranted.
Orbitals are mathematical functions that describe the probability of finding an electron in a specific region of space around the nucleus.
The s orbitals are spherical in shape, while p orbitals are dumbbell-shaped and oriented along three mutually perpendicular axes (px, py, pz).
Higher energy levels also include d orbitals and f orbitals, which have more complex shapes.
Argon’s electron configuration demonstrates how these orbitals are filled in a specific order, dictated by increasing energy levels.
This orderly filling leads to the stable octet configuration that defines Argon’s inert nature.
The orderly arrangement of electrons dictates Argon’s inertness. However, this isn’t the complete story of this fascinating element. While all Argon atoms share the same number of protons, they can differ in the number of neutrons, leading to the existence of isotopes.
Isotopes of Argon: Variations on a Theme
Isotopes introduce a subtle, yet significant, layer of complexity to our understanding of elements. While the atomic number defines an element, isotopes represent variations on that fundamental identity.
Defining Isotopes: Same Element, Different Mass
Isotopes are atoms of the same element that have the same number of protons (and therefore the same atomic number) but differ in the number of neutrons in their nuclei.
This difference in neutron number results in a difference in atomic mass. Think of it like building with LEGOs: you can build the same basic structure (same number of bricks of a certain color), but by adding more bricks of a different color, you change the overall weight of the structure.
Common Isotopes of Argon
Argon has several naturally occurring isotopes. The most abundant are Argon-40 (⁴⁰Ar), Argon-36 (³⁶Ar), and Argon-38 (³⁸Ar).
The numbers following "Argon" represent the mass number of the isotope, which is the sum of protons and neutrons in the nucleus.
For example, Argon-40 has 18 protons (as all Argon atoms do) and 22 neutrons (40 – 18 = 22). Similarly, Argon-36 has 18 protons and 18 neutrons.
The Impact of Isotopes on Atomic Mass
The existence of isotopes explains why the atomic mass of an element on the periodic table is often not a whole number. The atomic mass reported is a weighted average of the masses of all the naturally occurring isotopes, taking into account their relative abundance.
The most common isotope, Argon-40, contributes most significantly to Argon’s atomic mass. The other isotopes have a lesser impact, proportional to their presence in nature.
The differences in mass between isotopes of an element are small, but are sufficient enough for scientists to detect them using modern instruments like mass spectrometers.
Radioactive Argon Isotopes
While Argon-36, Argon-38 and Argon-40 are stable isotopes, some isotopes of Argon are radioactive. For example, Argon-39 (³⁹Ar) is a radioactive isotope with a half-life of 269 years.
Radioactive isotopes decay over time, emitting particles or energy. These radioactive isotopes of Argon are often produced by cosmic ray interactions within the Earth’s atmosphere.
These isotopes are useful in dating very old groundwater and determining the age of ice cores.
It is worth noting that radioactive isotopes often have medical and industrial applications, particularly in areas like imaging and cancer treatment.
Surprising Uses of Argon: Beyond Inertness
Argon, lauded for its inertness and chemical aloofness, might seem an unlikely candidate for widespread industrial and commercial application. Yet, this noble gas, far from being a mere bystander in the world of elements, plays a crucial role in diverse fields. From safeguarding critical welds to illuminating our homes, Argon’s unique properties are ingeniously harnessed. This leads to outcomes that defy its seemingly inactive nature.
Argon in Welding: A Shield Against Oxidation
One of Argon’s most significant applications lies in welding. Here, its inert nature becomes its greatest asset. In welding processes, high temperatures can cause metals to react with oxygen and nitrogen in the air.
This oxidation leads to weakened welds and corrosion. Argon, as a shielding gas, blankets the weld area.
It displaces the reactive atmospheric gases. By preventing these unwanted reactions, Argon ensures the creation of strong, clean, and durable welds. This is critical in industries ranging from automotive manufacturing to aerospace engineering.
Illuminating the World: Argon in Lighting
Argon finds extensive use in various lighting applications. In fluorescent lights, Argon is used along with mercury vapor. The combination creates the plasma that emits ultraviolet light. This light then excites the fluorescent coating on the bulb’s interior, producing visible light.
In incandescent light bulbs, Argon helps to extend the life of the filament. By filling the bulb with Argon, the rate of filament evaporation is reduced. This prevents the filament from quickly burning out. This leads to longer-lasting and more efficient bulbs.
Preservation Techniques: Protecting Sensitive Materials
Argon’s inertness also makes it invaluable in preservation. It prevents degradation caused by exposure to oxygen or other reactive substances. The food and wine industries leverage this property.
In wine preservation, Argon is used to displace the air in opened bottles. This prevents oxidation and maintains the wine’s flavor and aroma for longer. Argon is also used in the storage of sensitive materials.
It can be used to preserve historical documents. In manufacturing processes, it is also used to protect materials that are sensitive to atmospheric conditions.
Argon in Medicine: Specialized Applications
While less widely known, Argon has found niche applications in the medical field. Argon lasers are used in certain surgical procedures. They can be useful in ophthalmology for treating retinal disorders.
Argon gas is also used in cryotherapy. It can be used to destroy diseased tissue through freezing. Its inertness is helpful in certain types of gas-filled lenses used in eye surgery.
These applications highlight the specialized role Argon can play in advancing medical treatments.
In conclusion, Argon’s diverse applications, from welding to lighting to preservation and medicine, showcase the surprising utility of this noble gas. Its inertness, often perceived as a limitation, is the very key to its effectiveness. This makes Argon an indispensable element in a wide array of industries and technologies.
Argon’s applications, while diverse, all stem from its fundamental atomic properties. These properties, in turn, were only revealed through the meticulous work of dedicated scientists. Understanding how Argon was discovered offers valuable insight into the scientific process. It showcases how careful observation and persistent experimentation can unlock the secrets of the natural world.
Discovery of Argon: A Historical Perspective
The story of Argon’s discovery is a testament to the power of scientific curiosity and the importance of meticulous observation. It began in the late 19th century with a seemingly minor discrepancy that piqued the interest of two brilliant scientists: Lord Rayleigh and William Ramsay. Their collaborative efforts ultimately led to the isolation and identification of a new element. This discovery not only expanded the periodic table but also challenged existing understanding of the composition of air.
Lord Rayleigh’s Density Anomaly: The Spark of Discovery
Lord Rayleigh, a renowned British physicist, was deeply involved in precise measurements of gas densities. While preparing pure nitrogen samples, Rayleigh encountered a puzzling anomaly. He found that nitrogen extracted from air consistently had a higher density than nitrogen produced from chemical compounds like ammonia. This difference, though small, was persistent and statistically significant.
Rayleigh initially suspected experimental error, meticulously checking his procedures and equipment. However, the density difference persisted. This led him to hypothesize that air-derived nitrogen was contaminated with a heavier, unknown substance. This initial observation of a density anomaly served as the crucial first step in the discovery of Argon. His meticulousness turned a seemingly minor inconsistency into a major scientific breakthrough.
William Ramsay’s Role: Isolating the Unknown
Recognizing the significance of Rayleigh’s findings, William Ramsay, a Scottish chemist, joined the investigation. Ramsay, with his expertise in chemical separation, set out to isolate the mysterious substance responsible for the density discrepancy. He exposed air-derived nitrogen to heated magnesium, which readily reacts with nitrogen to form magnesium nitride.
Ramsay reasoned that if the nitrogen sample contained a heavier, unreactive gas, it would remain after the magnesium had consumed all the nitrogen. After repeated exposures to heated magnesium, Ramsay successfully isolated a small amount of a gas that was remarkably inert. This gas showed no signs of chemical reactivity, even under extreme conditions.
Experimental Techniques: Unveiling a New Element
The experimental techniques employed by Rayleigh and Ramsay were crucial in confirming the existence of Argon. Rayleigh’s precise density measurements provided the initial evidence. Ramsay’s chemical separation techniques allowed for the isolation of the unknown gas.
Spectroscopic analysis further confirmed the discovery. The isolated gas exhibited a unique atomic emission spectrum. This spectrum consisted of distinct lines that did not match any known element. This distinctive spectral fingerprint provided conclusive evidence that they had discovered a new element, which they named "Argon," derived from the Greek word argos, meaning "idle" or "inactive," reflecting its inert nature. The combination of precise measurements, careful chemical separation, and spectroscopic analysis cemented the discovery of Argon in the scientific community.
Rayleigh’s meticulousness turned what could have been a dismissed experimental error into a groundbreaking discovery. But the story doesn’t end with the identification of a new element. Understanding Argon goes beyond its mere existence; it requires delving into its atomic mass and how scientists use tools like spectroscopy to truly understand its nature.
Atomic Mass and Spectroscopy: Unlocking Further Secrets of Argon
While the atomic number provides the fundamental identity of an element, atomic mass and spectroscopy offer deeper insights into its composition and behavior. The atomic mass reflects the collective mass of protons and neutrons in an atom’s nucleus, influenced by the relative abundance of its isotopes. Spectroscopy, on the other hand, acts as a fingerprinting technique, revealing the unique spectral lines emitted by an element when energized. Together, these tools provide a powerful means of analyzing and identifying Argon.
The Nuances of Atomic Mass: A Weighted Average
Argon, like many elements, exists as a mixture of isotopes – atoms with the same number of protons but different numbers of neutrons. Each isotope contributes to the overall atomic mass, but not equally. The atomic mass reported on the periodic table is a weighted average of the masses of all naturally occurring isotopes, taking into account their relative abundance.
For example, Argon-40 is the most abundant isotope, making up the vast majority of naturally occurring Argon. Argon-36 and Argon-38 exist in smaller quantities. The atomic mass is not simply the average of 40, 36, and 38. Instead, it is calculated by multiplying the mass of each isotope by its fractional abundance and then summing the results. This ensures that the more prevalent isotopes have a greater influence on the overall atomic mass. The formula is:
Atomic Mass = (Mass of Isotope 1 × Abundance of Isotope 1) + (Mass of Isotope 2 × Abundance of Isotope 2) + …
This calculation provides a far more accurate representation of the "average" Argon atom found in nature.
Understanding the role of isotopic abundance is crucial for accurately interpreting atomic mass data. Variations in isotopic ratios can even be used in dating techniques and tracing the origins of Argon samples.
Spectroscopy: Deciphering the Light Signature of Argon
Spectroscopy is a powerful analytical technique that exploits the interaction between matter and electromagnetic radiation. When Argon atoms are energized, for example by heating them or passing an electric current through them, their electrons jump to higher energy levels.
As these electrons fall back to their original energy levels, they release energy in the form of light. This light is not continuous but rather consists of specific wavelengths, creating a unique spectral fingerprint for each element. These spectral lines can be observed using a spectroscope.
Each element possesses a unique set of spectral lines, analogous to a fingerprint. The specific wavelengths of light emitted by Argon are determined by the energy differences between its electron orbitals.
Applications of Argon Spectroscopy
By analyzing the wavelengths and intensities of these lines, scientists can not only identify the presence of Argon but also determine its concentration in a sample. This makes spectroscopy invaluable in various fields:
- Astronomy: Identifying Argon in stars and nebulae by analyzing the light they emit.
- Environmental Monitoring: Detecting Argon in air or water samples.
- Industrial Processes: Monitoring Argon levels in manufacturing processes.
- Plasma Research: Characterizing Argon plasmas used in various technological applications.
In essence, spectroscopy provides a non-destructive method for probing the electronic structure of Argon atoms, revealing information that would be inaccessible through other means.
Argon’s Spectral Lines: A Unique Fingerprint
Argon’s characteristic spectral lines are typically found in the visible and ultraviolet regions of the electromagnetic spectrum. These lines are well-defined and readily identifiable, making Argon relatively easy to detect and quantify using spectroscopic techniques. The precise wavelengths of these lines are precisely known and documented, allowing for accurate identification.
The intensity of each spectral line is directly proportional to the concentration of Argon present. This allows for quantitative analysis, enabling researchers to determine the amount of Argon in a sample with high precision. Because of its well-defined spectrum, Argon serves as a valuable calibration standard in many spectroscopic experiments.
By studying the atomic mass and spectral lines of Argon, scientists gain a comprehensive understanding of its atomic structure and behavior. These tools, combined with other analytical techniques, provide invaluable insights into this fascinating noble gas and its diverse applications.
FAQs: Argon’s Atomic Number & Uses
Here are some frequently asked questions about argon’s atomic number and its fascinating applications.
What exactly is Argon’s atomic number?
Argon’s atomic number is 18. This fundamental property defines argon and indicates that each argon atom has 18 protons in its nucleus.
Why is knowing the atomic number of argon important?
Knowing the atomic number of argon helps us understand its position on the periodic table and its chemical properties. All elements with an atomic number of 18 are, by definition, argon.
Where do we typically find argon?
Argon is a noble gas, making up about 1% of Earth’s atmosphere. It is produced industrially by the fractional distillation of liquid air.
What are some common uses of argon stemming from its properties?
Argon’s inert nature makes it valuable for applications like welding, where it shields metals from oxidation. It’s also used in incandescent light bulbs to prevent the filament from burning out, and in some medical procedures. The atomic number of argon dictates its electron configuration, which directly impacts these properties.
So, next time you see an ‘Ar’ on a welding tank or hear about those cool blue lights, remember we’ve uncovered some secrets about the atomic number argon and how it impacts our world. Pretty neat, right?