Electron configuration, a fundamental concept in quantum chemistry, provides the basis for understanding the properties of elements. These configurations are often visually represented using shell diagrams, with the octet rule dictating the stability achieved when the outermost shell contains eight electrons. The noble gas Argon, with its atomic number 18, exemplifies this stability, making the argon shell diagram a critical tool for visualizing its electron arrangement. This diagram reveals how Argon’s complete valence shell impacts its inertness and interactions with other elements, as extensively studied by Linus Pauling in his seminal work on chemical bonding.
Crafting the Ideal Article Layout: Argon Shell Diagrams Explained
This document outlines the optimal structure for an article focused on demystifying "argon shell diagrams." The structure prioritizes clarity, gradual learning, and caters to readers with varying levels of prior knowledge.
Understanding Your Audience and Setting Expectations
Before outlining the article, it’s important to define the target reader. Are they high school students, college undergraduates, or simply curious individuals? Assume a baseline understanding of basic atomic structure, but avoid assuming familiarity with shell diagrams specifically. The introduction must clearly state the article’s purpose: to explain what argon shell diagrams are, why they’re useful, and how to interpret them.
The Article Structure: Step-by-Step
The article should follow a logical progression, starting with fundamental concepts and gradually building towards more complex interpretations of the argon shell diagram.
Introduction: What are Shell Diagrams?
- Begin with a concise definition of a shell diagram (also known as a Bohr diagram). Explain it’s a simplified visual representation of an atom’s electron arrangement.
- Briefly contrast it with other atomic models (e.g., the Bohr model, the quantum mechanical model). Emphasize that shell diagrams are primarily for illustrating valence electrons and electronic configuration in a readily understandable manner.
- State the article’s objective: to specifically explain the argon shell diagram.
The Basics: Atomic Structure Refresher
Atoms, Elements, and the Periodic Table
- Quickly recap the fundamental components of an atom: protons, neutrons, and electrons. Explain their charges and locations.
- Define elements and their organization on the periodic table. Briefly mention atomic number (number of protons) and its significance.
- Relate this to argon: "Argon (Ar) is an element found on the periodic table with atomic number 18."
Electron Shells and Energy Levels
- Introduce the concept of electron shells (also known as energy levels).
- Explain that electrons occupy specific energy levels around the nucleus.
- Introduce the "2n2" rule: This rule helps determine the maximum number of electrons each shell can hold (where n is the shell number, counting outwards from the nucleus). Explain that the first shell (n=1) can hold up to 2 electrons, the second shell (n=2) up to 8 electrons, and the third shell (n=3) up to 18 electrons.
-
Use a table for clarity:
Shell Number (n) Maximum Electrons (2n2) 1 2 2 8 3 18 4 32
Argon: A Closer Look
Argon’s Atomic Number and Electron Configuration
- Reinforce that argon has an atomic number of 18, meaning it has 18 protons and, in a neutral atom, 18 electrons.
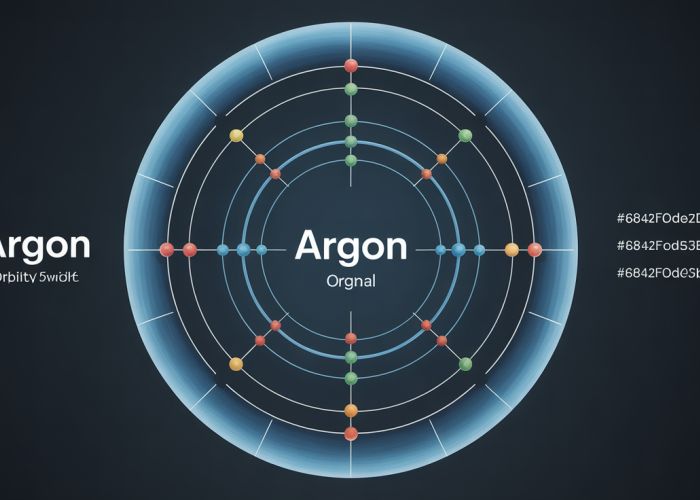
- Explain argon’s electron configuration: 2 electrons in the first shell, 8 electrons in the second shell, and 8 electrons in the third shell. (2, 8, 8).
Representing Argon’s Electron Configuration
- Visually represent the electron configuration with numbers corresponding to each shell: 2-8-8. This should be clearly displayed.
- Emphasize that the outermost shell, which is the valence shell, determines an atom’s chemical properties.
Constructing the Argon Shell Diagram
Step-by-Step Guide
- Draw the Nucleus: Represent the nucleus with a small circle or dot in the center. Write "Ar" (the symbol for argon) inside the circle. It is optional to include the number of protons (18) and neutrons (usually calculated based on the most common isotope) within the nucleus.
- Draw the Shells: Draw concentric circles around the nucleus representing the electron shells.
- Populate the Shells with Electrons:
- In the first shell (closest to the nucleus), place 2 electrons (represented as dots or small "x"s).
- In the second shell, place 8 electrons.
- In the third shell (the valence shell), place 8 electrons.
- Labeling (Optional): You can label each shell with its number (1, 2, 3) or its energy level (K, L, M).
Visual Example
- Include a clear, high-quality image or diagram of the complete argon shell diagram, clearly showing the nucleus and all three shells with their respective electrons. Ensure the electron placement is visually distinct.
Interpreting the Argon Shell Diagram
Valence Electrons and Chemical Inertness
- Define valence electrons as the electrons in the outermost shell.
- Explain that argon has 8 valence electrons, making it an octet.
- Connect the octet rule: Atoms "want" to have a full valence shell (usually 8 electrons) to be stable.
- Explain that because argon already has a full valence shell, it is very stable and unreactive. It is a noble gas, meaning it rarely forms chemical bonds with other elements.
Applications and Implications
- Briefly mention the significance of understanding shell diagrams for predicting chemical behavior.
- Give examples: Argon’s inertness makes it useful in applications like welding, lighting, and preserving artifacts, where a non-reactive atmosphere is required.
Common Mistakes and Misconceptions
- Addressing common mistakes in drawing shell diagrams: Incorrect number of electrons, incorrect shell filling order.
- Clarifying the limitations of shell diagrams: They are a simplified model and don’t accurately represent the complex quantum mechanical nature of electron behavior.
- Explaining that isotopes of argon will still have the same electron arrangement, even though they have a different number of neutrons.
Practice and Further Learning
- Include simple practice questions for the reader to test their understanding. For instance: "Draw the shell diagram for Neon (Ne)."
- Provide links to external resources for further learning about atomic structure and chemical bonding.
Argon Shell Diagrams: FAQs
Here are some frequently asked questions about argon shell diagrams to help clarify their use and interpretation.
What exactly is an argon shell diagram used for?
An argon shell diagram is primarily used to visually represent and understand the electronic structure of atoms, specifically focusing on the arrangement of electrons in different energy levels or shells. It’s a helpful tool for visualizing electron configurations.
How does an argon shell diagram differ from a standard Bohr model diagram?
While both diagrams illustrate electron arrangement, the argon shell diagram generally shows all electron shells and the number of electrons in each, potentially extending beyond the simplified view often used in introductory Bohr model examples. An argon shell diagram emphasizes the completion of the argon-like electronic structure in certain compounds.
What does it mean when a diagram shows an atom achieving an "argon-like" configuration?
Achieving an "argon-like" configuration means an atom has gained or lost electrons to have the same stable electron arrangement as the noble gas argon, with eight valence electrons. This stability is a driving force in chemical bonding, and the argon shell diagram helps visualize how atoms achieve this.
Why is the element argon so important in understanding these diagrams?
Argon’s stable electron configuration (2, 8, 8) serves as a benchmark for other atoms. Elements tend to react in ways that allow them to achieve this stable arrangement, making argon shell diagrams a valuable tool for understanding chemical reactivity and ion formation by visualizing if elements would gain or lose valence electrons.
So, there you have it – a deep dive into the argon shell diagram! Hopefully, you now have a much clearer picture of what it all means. Keep exploring and applying this knowledge to understand even more complex chemical concepts. Happy learning!