Understanding the ammonium cation formula is foundational in chemistry. The Lewis Structure, a key concept in understanding molecular geometry, provides a visual representation of how atoms bond to form the ammonium cation (NH₄⁺). This ammonium cation is a polyatomic ion, its chemical behavior significantly studied in solutions by organizations like the International Union of Pure and Applied Chemistry (IUPAC). Its properties are vital in contexts such as fertilizers, and you can easily test it in a laboratory setting by using the Nessler’s reagent.
Understanding the Best Article Layout: "Unlock the Ammonium Cation Formula: A Simple Guide"
This explanation details an effective article layout for the topic "Unlock the Ammonium Cation Formula: A Simple Guide," focusing on the main keyword "ammonium cation formula." The layout prioritizes clarity, understanding, and educational value for readers who may not have prior knowledge of chemistry.
Introduction: Setting the Stage
The introductory paragraph is crucial for engaging the reader and establishing the article’s purpose. It should:
- Briefly introduce the concept of ions and their importance.
- Mention the significance of the ammonium cation in various fields (e.g., agriculture, fertilizers, chemical reactions).
- Clearly state the article’s aim: to demystify the ammonium cation formula and provide a simple, understandable explanation.
- Include the keyword "ammonium cation formula" naturally within the first few sentences.
What is an Ion? A Foundation for Understanding
Before diving into the specific formula, a brief explanation of ions is necessary.
Defining Ions: Atoms with Charge
- Explain that atoms are normally neutral, possessing an equal number of protons (positive charge) and electrons (negative charge).
- Define an ion as an atom or molecule that has gained or lost electrons, resulting in a net electrical charge.
- Distinguish between cations (positively charged ions, formed by losing electrons) and anions (negatively charged ions, formed by gaining electrons).
Why Ions Matter: Importance in Chemistry
- Highlight the crucial role of ions in chemical bonding, particularly ionic bonding.
- Mention their involvement in various chemical reactions and biological processes.
- Provide a simple example of an ionic compound (e.g., table salt, NaCl) and explain how it’s formed from ions.
Decoding the Ammonium Cation Formula (NH₄⁺)
This section forms the core of the article, thoroughly explaining the ammonium cation formula.
Breaking Down the Formula: Element by Element
- Identify the elements present in the ammonium cation: Nitrogen (N) and Hydrogen (H).
- Explain the significance of the subscript "4": it indicates that there are four hydrogen atoms bonded to one nitrogen atom.
The Plus Sign (+): Understanding the Charge
- Explain that the "+" sign signifies that the ammonium ion carries a positive charge.
- Detail how this positive charge arises: nitrogen has formed four covalent bonds with hydrogen atoms. Nitrogen needs three bonds to be neutral, and forming one more makes the overall complex positively charged.
- Emphasize that the charge is associated with the entire ammonium ion, not just a single atom within it.
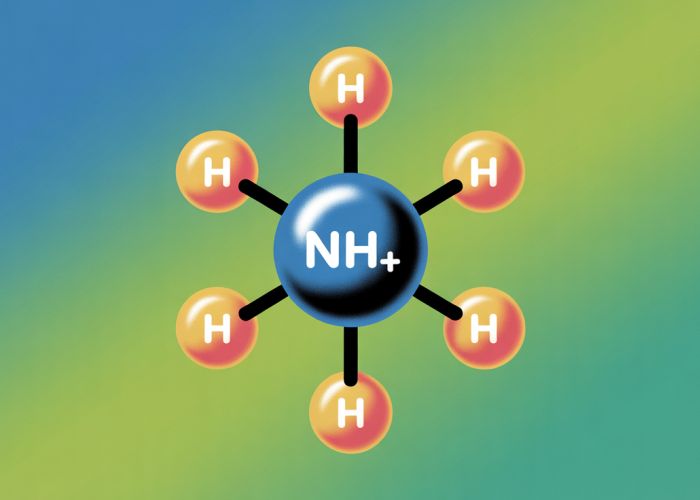
Visual Representation: A Diagram
Including a simple diagram or illustration of the ammonium ion (NH₄⁺) can significantly aid understanding. The diagram should:
- Visually depict the nitrogen atom bonded to four hydrogen atoms.
- Clearly show the positive charge associated with the entire ion.
- Use clear and concise labels for each atom and the overall charge.
The Ammonium Cation in Context: Examples and Applications
Providing real-world examples enhances the educational value of the article.
Ammonium Compounds: Common Examples
Presenting a list of common ammonium compounds with their formulas and uses is beneficial.
| Compound | Formula | Common Use(s) |
|---|---|---|
| Ammonium Chloride | NH₄Cl | Dry cell batteries, fertilizers, soldering flux |
| Ammonium Nitrate | NH₄NO₃ | Fertilizers, explosives |
| Ammonium Sulfate | (NH₄)₂SO₄ | Fertilizers, soil amendment |
| Ammonium Phosphate | (NH₄)₃PO₄ | Fertilizers |
| Ammonium Bicarbonate | NH₄HCO₃ | Baking powder |
Applications in Agriculture: The Role of Fertilizers
- Explain how ammonium compounds are used as fertilizers to provide nitrogen, an essential nutrient for plant growth.
- Discuss the benefits and potential drawbacks of using ammonium-based fertilizers.
Applications in Other Industries: Beyond Agriculture
- Briefly mention other industries where ammonium compounds are used, such as:
- Cleaning products (ammonium hydroxide).
- Pharmaceuticals.
- Textile manufacturing.
Common Mistakes and Misconceptions
Addressing common misunderstandings strengthens the article’s educational impact.
Confusing Ammonium with Ammonia
- Clearly differentiate between ammonium (NH₄⁺, an ion) and ammonia (NH₃, a neutral molecule).
- Explain that ammonia can gain a proton (H⁺) to form the ammonium ion.
Thinking the Charge Belongs to a Specific Atom
- Reiterate that the positive charge belongs to the entire ammonium ion, not just the nitrogen or a specific hydrogen atom.
- Use an analogy (e.g., a team effort) to illustrate this concept.
Misunderstanding the Bonding
- Clarify that the bonds between nitrogen and hydrogen are covalent bonds.
- Explain that while the overall ion carries a positive charge, the bonds themselves are formed by sharing electrons.
Frequently Asked Questions About Ammonium
Here are some common questions regarding the ammonium cation and its formula. We hope these answers provide clarity.
What exactly is the ammonium cation?
The ammonium cation is a positively charged polyatomic ion formed when ammonia (NH₃) accepts a proton (H⁺). It’s essentially ammonia with an extra hydrogen atom attached, giving it a +1 charge.
What is the ammonium cation formula?
The ammonium cation formula is NH₄⁺. This represents one nitrogen atom and four hydrogen atoms, with the entire group carrying a single positive charge. This formula is crucial for understanding chemical reactions involving ammonium.
Why does the ammonium cation have a positive charge?
The positive charge arises because a hydrogen ion (H⁺), which has a +1 charge, bonds to the neutral ammonia molecule (NH₃). Since ammonia is neutral and the hydrogen ion has a +1 charge, the resulting ammonium ion (NH₄⁺) also has a +1 charge. The ammonium cation formula reflects this.
Where might I encounter the ammonium cation in everyday life?
You’ll find the ammonium cation in many fertilizers, cleaning products, and certain types of batteries. It plays a vital role in various chemical processes, including nitrogen cycling in the environment. Understanding the ammonium cation formula helps in recognizing its presence in these applications.
So, there you have it – a simple guide to unlocking the ammonium cation formula! Hopefully, this helped clarify things a bit. Best of luck as you continue learning!