Acetylsalicylic acid, commonly known as aspirin, exhibits significant polarity. The extent of acetylsalicylic acid polarity affects its solubility in various solvents, a characteristic critical in pharmaceutical formulations. Understanding acetylsalicylic acid polarity requires knowledge of its molecular structure and the influence of the carboxyl and ester functional groups. Furthermore, chromatographic techniques can be used to quantitatively analyze acetylsalicylic acid polarity, providing valuable data for researchers at institutions such as Pfizer and Merck studying its behavior.
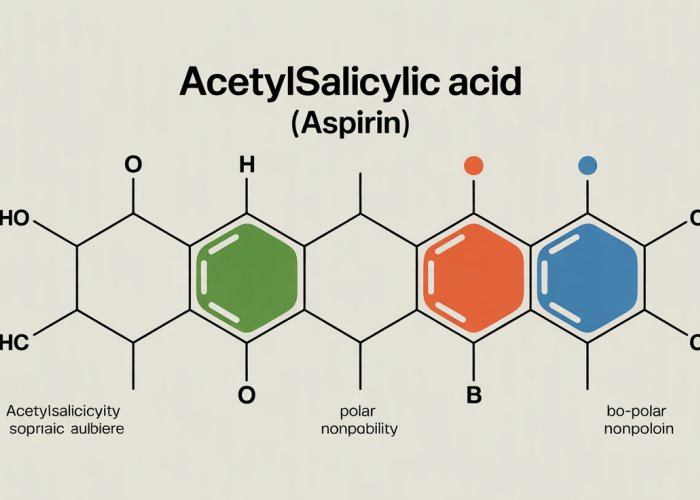
Acetylsalicylic acid, more commonly known as aspirin, is a ubiquitous medication found in households worldwide. Its primary applications range from alleviating minor aches and pains to preventing serious cardiovascular events. Understanding the multifaceted nature of aspirin is crucial, extending beyond its immediate therapeutic effects. A key aspect of this understanding lies in appreciating its polarity—a fundamental property that dictates its behavior within the complex environment of the human body.
Acetylsalicylic Acid: A Brief Overview
Aspirin is a synthetic derivative of salicylic acid, belonging to the class of drugs known as nonsteroidal anti-inflammatory drugs (NSAIDs). Its medicinal applications are wide-ranging, including:
- Analgesic: Relief from mild to moderate pain.
- Antipyretic: Reduction of fever.
- Anti-inflammatory: Mitigation of inflammation.
- Antiplatelet: Inhibition of blood clot formation, making it useful in preventing heart attacks and strokes.
Polarity: A Cornerstone of Chemical and Pharmacological Behavior
In chemistry, polarity refers to the unequal sharing of electrons in a chemical bond, leading to a separation of electric charge within a molecule. This charge distribution creates a dipole moment, a vector quantity that describes the magnitude and direction of the polarity.
The polarity of a molecule significantly influences its interactions with other molecules, its solubility in different solvents, and its overall behavior in chemical and biological systems. In pharmacology, understanding a drug’s polarity is paramount because it affects:
- Its ability to cross cell membranes.
- Its distribution throughout the body.
- Its binding affinity to target proteins.
- Its metabolism and excretion.
The Significance of Understanding Aspirin’s Polarity
Acetylsalicylic acid possesses a unique chemical structure that imparts a specific degree of polarity. This polarity is not merely a static property; it is a dynamic characteristic influenced by various factors, such as pH and the surrounding solvent environment.
Grasping the intricacies of aspirin’s polarity is vital for several reasons:
- Predicting its Solubility: Polarity determines how well aspirin dissolves in different fluids, affecting its absorption from the gastrointestinal tract.
- Understanding its Distribution: Polarity influences how aspirin is distributed throughout the body, impacting its access to target tissues.
- Optimizing Drug Delivery: By manipulating the polarity of aspirin or its formulations, scientists can enhance its delivery to specific sites in the body.
- Enhancing Therapeutic Efficacy: A deeper understanding of polarity can lead to the development of more effective aspirin-based therapies with fewer side effects.
In essence, unraveling the polar nature of acetylsalicylic acid is indispensable for pharmaceutical scientists, healthcare professionals, and anyone seeking a comprehensive understanding of this widely used medication.
Acetylsalicylic acid possesses a unique molecular structure which subsequently dictates its behavior within biological systems. Before we can delve into the specifics of aspirin’s polarity, however, it is essential to establish a firm understanding of the fundamental principles that govern polarity in chemical compounds. This involves clarifying key definitions and exploring the underlying concepts that determine how molecules interact with each other and their environment.
Polarity Demystified: Core Principles and Definitions
Defining Polarity in Chemical Compounds
At its core, polarity in a chemical compound describes an uneven distribution of electron density. This unevenness creates a partial positive charge (δ+) on one part of the molecule and a partial negative charge (δ-) on another. It’s crucial to understand that polarity is not an all-or-nothing phenomenon; rather, it exists on a spectrum.
A molecule can be nonpolar, meaning the electron distribution is essentially equal, or polar, indicating a significant charge separation. This charge separation dictates how a molecule interacts with its surroundings, influencing its physical properties and chemical reactivity.
Electronegativity: The Driving Force Behind Polarity
The primary driver of polarity is electronegativity, a measure of an atom’s ability to attract electrons in a chemical bond. When two atoms with differing electronegativities form a bond, the more electronegative atom will pull the shared electrons closer to itself.
This creates an electron-rich region around the more electronegative atom and an electron-deficient region around the less electronegative one. Common examples of highly electronegative atoms include oxygen, nitrogen, and fluorine.
Bond Dipoles: The Building Blocks of Molecular Polarity
The unequal sharing of electrons in a chemical bond creates a bond dipole, a vector quantity representing the magnitude and direction of the charge separation. The magnitude of the bond dipole is directly proportional to the difference in electronegativity between the two atoms.
The direction of the dipole points from the positive end (δ+) to the negative end (δ-). Understanding bond dipoles is essential for predicting the overall polarity of a molecule.
Dipole Moment: Quantifying Molecular Polarity
While bond dipoles describe the polarity of individual bonds, the dipole moment (μ) is a measure of the overall polarity of the entire molecule. It is the vector sum of all the individual bond dipoles within the molecule.
A molecule with a significant dipole moment is considered polar, while a molecule with a dipole moment of zero is nonpolar. The dipole moment is typically measured in Debyes (D). The symmetry of a molecule plays a crucial role; even if individual bonds are polar, a symmetrical arrangement can cancel out the bond dipoles, resulting in a nonpolar molecule.
Functional Groups: Key Contributors to Polarity
Certain functional groups significantly influence a molecule’s polarity due to the presence of highly electronegative atoms or specific bonding arrangements.
Carboxylic acid groups (-COOH), present in aspirin, contain both a carbonyl group (C=O) and a hydroxyl group (-OH), both of which are highly polar. The oxygen atoms in these groups strongly attract electrons, creating a significant dipole moment.
Ester groups (-COOR), also present in aspirin, feature a carbon atom double-bonded to an oxygen and single-bonded to another oxygen. Like carboxylic acids, esters are polar due to the presence of these electronegative oxygen atoms. The arrangement of these atoms contributes to a distinct dipole moment within the ester functional group. These functional groups play a pivotal role in determining aspirin’s overall polarity and its subsequent interactions within biological systems.
Acetylsalicylic acid possesses a unique molecular structure which subsequently dictates its behavior within biological systems. Before we can delve into the specifics of aspirin’s polarity, however, it is essential to establish a firm understanding of the fundamental principles that govern polarity in chemical compounds. This involves clarifying key definitions and exploring the underlying concepts that determine how molecules interact with each other and their environment.
Acetylsalicylic Acid’s Molecular Polarity: A Detailed Analysis
Now that we have explored the core principles of polarity, we can apply that knowledge to acetylsalicylic acid itself. By carefully examining its molecular structure, we can identify the regions of high electron density and understand how its functional groups contribute to its overall polarity. This analysis will illuminate how acetylsalicylic acid interacts with its environment and exerts its effects within the body.
Dissecting the Molecular Structure of Acetylsalicylic Acid
Acetylsalicylic acid (aspirin) is comprised of a benzene ring substituted with both an acetyl group and a carboxylic acid group. The benzene ring, a six-carbon cyclic structure with alternating single and double bonds, forms the foundational backbone.
Attached to this ring are the two key functional groups that significantly influence the molecule’s properties: the carboxylic acid (-COOH) and the ester group (-OCOCH3). The strategic placement of these groups dictates the regions of varying electron density within the molecule.
Identifying Polar Regions Within Aspirin
The polar regions of acetylsalicylic acid are primarily concentrated around the oxygen atoms present in the carboxylic acid and ester groups. Oxygen, being a highly electronegative atom, strongly attracts electrons, resulting in partial negative charges (δ-) on these oxygen atoms.
Consequently, the carbon and hydrogen atoms directly bonded to these oxygens bear partial positive charges (δ+).
This charge separation creates localized dipoles, contributing to the overall polarity of the molecule. The hydroxyl group (-OH) within the carboxylic acid is a particularly prominent polar region due to the substantial electronegativity difference between oxygen and hydrogen.
The Influential Roles of Carboxylic Acid and Ester Groups
Both the carboxylic acid and ester groups exert a considerable influence on the overall polarity of acetylsalicylic acid, but they do so in slightly different ways.
The carboxylic acid group is strongly polar due to the presence of the hydroxyl group (-OH) and the carbonyl group (C=O). The hydroxyl group, as mentioned, features a highly polarized O-H bond, making it a significant contributor to hydrogen bonding.
The carbonyl group, with its double bond between carbon and oxygen, also creates a strong dipole moment.
The ester group, while also polar, is somewhat less so than the carboxylic acid. The carbonyl group (C=O) in the ester still contributes to polarity, but the presence of an alkoxy group (-OR) instead of a hydroxyl group reduces the overall dipole moment compared to the carboxylic acid.
Electron Distribution and Dipole Moment
The uneven distribution of electrons in acetylsalicylic acid results in a net dipole moment. This dipole moment is a vector quantity that reflects both the magnitude and direction of the overall polarity.
The direction of the dipole moment points from the regions of positive charge towards the regions of negative charge.
In acetylsalicylic acid, the dipole moment is primarily oriented towards the oxygen atoms of the carboxylic acid and ester groups. While the benzene ring itself is nonpolar, its substitution with these polar functional groups significantly alters the electron distribution. Computational chemistry methods can be used to calculate and visualize the electron density distribution and dipole moment of the molecule, providing a more quantitative understanding of its polar character.
Factors Modulating Acetylsalicylic Acid Polarity
Having dissected the intrinsic polarity of acetylsalicylic acid based on its molecular structure, it’s crucial to recognize that this polarity isn’t static. Several external and internal factors can modulate or influence the expression of this polarity, ultimately affecting aspirin’s behavior within biological systems and its interactions with other molecules. These factors include pH, hydrogen bonding, solvent interactions, and, to a lesser extent, van der Waals forces.
The Impact of pH on Acetylsalicylic Acid’s Charge and Polarity
The pH of the surrounding environment significantly impacts acetylsalicylic acid’s charge and, consequently, its polarity. Aspirin contains a carboxylic acid group (-COOH), which can donate a proton (H+) in aqueous solutions, especially at higher pH levels.
When the pH is below the pKa of the carboxylic acid group (around 3.5), the carboxylic acid predominantly exists in its protonated form (-COOH), making the molecule relatively less polar.
However, as the pH increases above the pKa, the carboxylic acid group begins to deprotonate, forming a negatively charged carboxylate ion (-COO-).
This ionization increases the molecule’s overall polarity due to the presence of a negative charge, enhancing its water solubility and altering its interactions with other molecules. The degree of ionization, and therefore the polarity, is directly proportional to the pH.
Hydrogen Bonding Capacity of Aspirin
Hydrogen bonds are a type of intermolecular force that occurs between a hydrogen atom covalently bonded to a highly electronegative atom (such as oxygen) and another electronegative atom.
Acetylsalicylic acid contains several oxygen atoms within its carboxylic acid and ester groups, making it capable of forming hydrogen bonds with other polar molecules, such as water.
These hydrogen bonds can influence aspirin’s solubility, stability, and interactions with biological targets. The ability to form hydrogen bonds enhances its interactions with aqueous environments and polar biomolecules.
The strength and number of hydrogen bonds formed depend on the availability of hydrogen bond donors (H atoms) and acceptors (O atoms) in both aspirin and the surrounding medium.
Solvent Interactions and Polarity Expression
The solvent in which acetylsalicylic acid is dissolved plays a critical role in determining its solubility and, indirectly, the expression of its polarity. The principle of "like dissolves like" dictates that polar solvents tend to dissolve polar solutes, while nonpolar solvents dissolve nonpolar solutes.
-
Water: A highly polar solvent, water readily dissolves ionized acetylsalicylic acid (carboxylate form) due to favorable dipole-dipole interactions and hydrogen bonding.
-
Ethanol: Being less polar than water, ethanol can still dissolve acetylsalicylic acid to a reasonable extent due to its intermediate polarity.
-
Ether: A relatively nonpolar solvent, ether dissolves acetylsalicylic acid to a lesser extent compared to water or ethanol.
-
Hexane: As a nonpolar solvent, hexane exhibits minimal interaction with acetylsalicylic acid, resulting in low solubility.
The solubility of aspirin in a given solvent is a reflection of the compatibility of their polarities.
The Subtle Influence of van der Waals Forces
Van der Waals forces are weak, short-range intermolecular forces that arise from temporary fluctuations in electron distribution, creating temporary dipoles. These forces include London dispersion forces, dipole-dipole interactions, and dipole-induced dipole interactions.
While van der Waals forces exist between acetylsalicylic acid molecules and surrounding molecules, their contribution to modulating aspirin’s overall polarity is generally minor compared to pH, hydrogen bonding, and solvent effects, especially in aqueous environments.
However, in nonpolar environments, these forces can become more significant in influencing aspirin’s interactions with other nonpolar molecules. They contribute to the overall intermolecular interactions, but they don’t drastically alter the fundamental polarity of the molecule.
In conclusion, while acetylsalicylic acid possesses an inherent polarity dictated by its molecular structure, this polarity is dynamic and subject to modulation by environmental factors. pH, hydrogen bonding, and solvent interactions play a dominant role in influencing aspirin’s behavior. While van der Waals forces exert a weaker, yet still present, influence.
Understanding these modulating factors is crucial for predicting aspirin’s solubility, distribution, and efficacy within biological systems.
Factors influencing aspirin’s polarity—like pH, hydrogen bonding, and solvent interactions—set the stage for understanding how these properties manifest in its solubility. After all, a drug’s ability to dissolve in different environments directly impacts its bioavailability, efficacy, and overall behavior within the body.
Polarity and Solubility: Predicting Aspirin’s Dissolution Behavior
The interaction between polarity and solubility is a cornerstone of pharmaceutical science, and it dictates how effectively a drug like aspirin can be delivered and utilized by the body. Solubility, at its core, is the ability of a substance (the solute, in this case, aspirin) to dissolve in a solvent to form a solution. Aspirin’s inherent polarity, modulated by the factors discussed previously, plays a crucial role in determining its affinity for different solvents.
The Interplay of Polarity and Solubility
The fundamental rule governing solubility is that “like dissolves like.”
This principle implies that polar substances tend to dissolve in polar solvents, while nonpolar substances dissolve best in nonpolar solvents. This behavior arises from the intermolecular forces that govern interactions between molecules.
Polar solvents, like water, can effectively solvate polar solutes, such as ionized aspirin, through dipole-dipole interactions and hydrogen bonding.
Nonpolar solvents, like hexane, lack these capabilities and are thus better suited for dissolving nonpolar solutes through weaker van der Waals forces.
Acetylsalicylic Acid’s Solubility Profile
Acetylsalicylic acid, possessing both polar (carboxylic acid, ester) and nonpolar (aromatic ring) regions, exhibits amphiphilic characteristics, meaning it has both hydrophobic and hydrophilic properties. This unique structure influences its solubility in various solvents.
Solubility in Water
Water, a highly polar solvent, interacts favorably with the polar regions of aspirin. However, aspirin’s solubility in water is limited due to the presence of the nonpolar aromatic ring.
The ionization of the carboxylic acid group at higher pH levels significantly enhances its water solubility by introducing a negative charge and increasing its polarity.
Solubility in Organic Solvents
Organic solvents present a more varied scenario.
In polar organic solvents like ethanol, aspirin exhibits good solubility due to favorable interactions with both its polar and nonpolar regions.
In nonpolar solvents like hexane, aspirin’s solubility is considerably lower because the nonpolar aromatic ring is the only portion of the molecule that interacts favorably with the solvent. The polar functional groups within aspirin do not solvate well in nonpolar environments, leading to diminished solubility.
Implications of the "Like Dissolves Like" Principle
The "like dissolves like" principle is not just a theoretical concept; it has practical implications for drug formulation and delivery.
Understanding aspirin’s solubility profile allows pharmaceutical scientists to select appropriate solvents for formulating liquid medications or to design solid dosage forms that promote rapid dissolution in the gastrointestinal tract.
For example, if a rapid onset of action is desired, aspirin might be formulated with excipients that enhance its solubility in the aqueous environment of the stomach. Conversely, if a sustained-release formulation is needed, the drug might be formulated in a manner that slows down its dissolution rate.
In conclusion, the interplay between polarity and solubility is a critical determinant of aspirin’s behavior. By understanding how the "like dissolves like" principle governs these interactions, we can better predict and control aspirin’s dissolution, absorption, and ultimately, its therapeutic efficacy.
The previous discussion highlighted how polarity influences a drug’s solubility in various solvents. But going beyond qualitative descriptions, is there a way to quantify a molecule’s preference for a particular environment? The answer lies in understanding lipophilicity and its quantitative expression through the partition coefficient.
Quantifying Lipophilicity: Understanding the Partition Coefficient (LogP)
While solubility provides insight into how well a substance dissolves in a specific solvent, lipophilicity offers a more comprehensive view of its affinity for lipid (fat-like) environments relative to aqueous (water-based) ones.
The partition coefficient (P) and its logarithmic form (LogP) are essential tools for quantifying this property, providing valuable information for predicting a drug’s behavior within the complex biological milieu.
Defining the Partition Coefficient (P)
The partition coefficient (P) is defined as the ratio of a compound’s concentration at equilibrium in a two-phase system consisting of two immiscible solvents, typically n-octanol and water. n-octanol serves as a mimic for the lipid environment of biological membranes, while water represents the aqueous environment of the body.
Mathematically, P is expressed as:
P = [Compound]octanol / [Compound]water
This ratio indicates how much more or less soluble the compound is in octanol compared to water.
Interpreting LogP Values: A Guide
Because partition coefficients can span several orders of magnitude, it’s more practical to use the logarithm of P, denoted as LogP.
LogP = log10(P)
Interpreting LogP values is straightforward:
-
A LogP of 0 indicates equal affinity for both octanol and water.
-
A positive LogP value suggests a higher affinity for the octanol phase, indicating lipophilicity. The higher the value, the more lipophilic the compound.
-
A negative LogP value indicates a higher affinity for the water phase, suggesting hydrophilicity. The more negative the value, the more hydrophilic the compound.
For example, a LogP of 2 means the compound is 100 times more soluble in octanol than in water.
Conversely, a LogP of -1 indicates it is 10 times more soluble in water than in octanol.
Determining LogP Values: Experimental and Computational Methods
LogP values can be determined through experimental methods or predicted computationally.
Experimental Determination: The shake-flask method is a common technique. It involves dissolving the compound in a mixture of octanol and water, allowing the system to reach equilibrium, and then measuring the concentration of the compound in each phase using techniques like UV-Vis spectroscopy or HPLC.
Computational Prediction: Numerous software tools and algorithms estimate LogP based on the compound’s molecular structure. These methods rely on fragment-based approaches, where the LogP is calculated by summing the contributions of individual atoms or molecular fragments, and correction factors.
Computational methods offer a rapid and cost-effective alternative to experimental measurements, especially during the early stages of drug discovery.
Significance of LogP in Drug Development
LogP is a critical parameter in drug development due to its influence on various aspects of a drug’s journey through the body.
-
Absorption: Lipophilic drugs (high LogP) tend to be better absorbed across biological membranes, such as the gastrointestinal tract, because they can more easily diffuse through the lipid bilayer.
-
Distribution: LogP affects how a drug distributes to different tissues and organs. Highly lipophilic drugs may accumulate in fatty tissues, potentially leading to prolonged effects or toxicity.
-
Metabolism: The liver’s ability to metabolize a drug can be influenced by its lipophilicity. More lipophilic drugs might undergo different metabolic pathways.
-
Excretion: Hydrophilic drugs (low LogP) are generally more readily excreted through the kidneys.
-
Drug-Target Interactions: A drug’s lipophilicity can impact its ability to bind to its target receptor or enzyme, particularly if the binding site contains hydrophobic pockets.
LogP’s Impact on Drug Delivery
LogP plays a crucial role in drug delivery strategies, impacting both the formulation and the route of administration.
-
Formulation: In formulating a drug, considerations around LogP can influence the choice of excipients, solubilizers, and delivery systems. For instance, liposomes might be used to encapsulate and deliver highly lipophilic drugs, enhancing their water solubility and bioavailability.
-
Route of Administration: The suitability of different routes of administration, like oral, intravenous, or transdermal, depends on the drug’s LogP. Transdermal delivery, for example, is favored for drugs with moderate lipophilicity that can permeate the skin’s lipid layers.
By understanding and optimizing a drug’s LogP, pharmaceutical scientists can improve its bioavailability, efficacy, and overall therapeutic outcome. Balancing lipophilicity and hydrophilicity is often the key to successful drug design.
Quantifying lipophilicity, through LogP, allows us to predict a drug’s behavior in simplified systems. But how does this translate to the complex environment of the human body? The influence of polarity extends to every stage of a drug’s journey, dictating its absorption, distribution, metabolism, and excretion, as well as its interactions with biological targets.
Practical Implications: Polarity and Acetylsalicylic Acid’s Journey in the Body
The polarity of acetylsalicylic acid profoundly influences its pharmacokinetic profile, governing its Absorption, Distribution, Metabolism, and Excretion (ADME) processes. Understanding these interactions is crucial for optimizing drug efficacy and minimizing adverse effects.
Absorption: Crossing Biological Barriers
Absorption, the process by which a drug enters the bloodstream, is heavily influenced by polarity. Acetylsalicylic acid, with its moderate polarity, can passively diffuse across cell membranes.
However, the rate and extent of absorption depend on the pH of the environment. In the acidic environment of the stomach, aspirin exists predominantly in its non-ionized, more lipophilic form, facilitating absorption through the gastric mucosa.
Conversely, in the more alkaline environment of the small intestine, a greater proportion of aspirin becomes ionized, reducing its lipophilicity and thus its ability to passively diffuse across the intestinal lining. This complex interplay highlights how polarity, modulated by environmental pH, dictates the rate and location of drug absorption.
Distribution: Navigating the Body
Once absorbed, acetylsalicylic acid is distributed throughout the body via the bloodstream. The distribution process is also influenced by polarity.
Aspirin’s moderate polarity allows it to readily distribute into both aqueous and lipid compartments. However, its binding to plasma proteins, such as albumin, can restrict its distribution.
This binding is influenced by the drug’s polarity and the hydrophobic regions on the protein surface. The extent of protein binding affects the amount of free drug available to interact with its target and undergo metabolism or excretion.
Metabolism: Transformation and Detoxification
Metabolism, primarily occurring in the liver, involves enzymatic modification of the drug molecule. Acetylsalicylic acid undergoes hydrolysis, yielding salicylic acid and acetic acid.
The polarity of aspirin and its metabolites influences their susceptibility to further metabolic transformations, such as conjugation reactions. These reactions typically involve the addition of polar groups, increasing water solubility and facilitating excretion.
Excretion: Elimination from the Body
Excretion, the process by which the drug and its metabolites are eliminated from the body, predominantly occurs via the kidneys. The polarity of the compounds plays a crucial role in determining their renal clearance.
More polar metabolites are more readily excreted in the urine. The kidneys filter drugs from the blood, but lipophilic compounds can be reabsorbed back into the bloodstream.
Polar compounds, however, are less likely to be reabsorbed and are efficiently eliminated in the urine. The balance between filtration, reabsorption, and secretion dictates the overall renal clearance of acetylsalicylic acid and its metabolites.
Polarity and Drug-Target Interactions: Binding and Efficacy
The efficacy of acetylsalicylic acid hinges on its ability to bind to its target enzyme, cyclooxygenase (COX), inhibiting the production of prostaglandins. The drug-target interaction is dictated by the shape and electronic properties of both the drug and the target.
The polar regions of aspirin, such as the carboxylic acid group, can form hydrogen bonds with amino acid residues in the active site of COX. While hydrophobic regions on aspirin can interact with complementary regions on the enzyme through van der Waals forces.
These intermolecular interactions, influenced by the polarity distribution within the molecule, determine the affinity and selectivity of aspirin for its target.
Polarity in Pharmaceutical Formulation and Dosage Form Design
The polarity of acetylsalicylic acid has implications for pharmaceutical formulation and dosage form design. The drug’s moderate polarity affects its compatibility with various excipients, influencing the choice of solvents, binders, and coating materials.
For example, the formulation of aspirin tablets requires excipients that promote drug dissolution and absorption. The drug’s polarity also affects its stability in different dosage forms.
Aspirin is susceptible to hydrolysis, particularly in the presence of moisture. Therefore, formulations must be designed to minimize exposure to water and enhance stability. The design of controlled-release formulations also considers the drug’s polarity to modulate its release rate and duration of action.
Frequently Asked Questions About Acetylsalicylic Acid Polarity
Here are some common questions regarding the polarity of acetylsalicylic acid, also known as Aspirin, to help you better understand its properties.
Is acetylsalicylic acid polar or nonpolar?
Acetylsalicylic acid has both polar and nonpolar regions within its molecule. The presence of the carboxyl group (-COOH) and ester group (-COOR) contributes to its polar character. However, the benzene ring adds a nonpolar component.
How does acetylsalicylic acid polarity affect its solubility?
The presence of both polar and nonpolar groups means acetylsalicylic acid is somewhat soluble in both polar solvents (like water) and nonpolar solvents (like ethanol). Its solubility is enhanced by the polar regions, though it is not highly soluble in water alone.
Does acetylsalicylic acid polarity influence its drug delivery?
Yes, the acetylsalicylic acid polarity is an important factor. The balance of polar and nonpolar characteristics allows it to cross biological membranes to some extent. However, its limited water solubility can sometimes affect its absorption and distribution.
Why is understanding acetylsalicylic acid polarity important?
Knowing the acetylsalicylic acid polarity helps predict its behavior in different chemical environments and biological systems. It provides valuable insights into its solubility, interactions with other molecules, and overall effectiveness as a medication.
So, there you have it! Hopefully, you now have a better grasp of acetylsalicylic acid polarity and how it all works. Go forth and use this knowledge wisely!