Infrared Spectroscopy, a technique utilized across organic chemistry, provides valuable insights into molecular structures. Acetone, a common solvent and chemical reagent, possesses a unique spectral fingerprint. The acetone IR spectrum, therefore, holds crucial data for identifying and analyzing the compound. Spectral analysis software enhances the interpretation of these complex spectra, aiding researchers in understanding the vibrational modes present in acetone’s molecular bonds.
Acetone, a ubiquitous organic solvent, plays a critical role in various industrial, laboratory, and even household applications. From nail polish remover to chemical synthesis, its versatility is undeniable. Understanding its molecular fingerprint is crucial for quality control, identification, and research purposes.
Infrared (IR) spectroscopy offers a powerful, non-destructive method for probing the vibrational modes of molecules. By analyzing the absorption of infrared radiation, we can gain valuable insights into the functional groups present within a molecule. This information allows us to identify and characterize substances with remarkable accuracy.
Acetone: A Versatile Chemical Compound
Acetone (CH3COCH3), also known as propanone, is the simplest ketone. It is a colorless, volatile, and flammable liquid. Its excellent solvent properties make it ideal for dissolving a wide range of organic materials.
Its applications span across diverse sectors:
- Industrial Solvent: Used in paints, varnishes, resins, and adhesives.
- Laboratory Reagent: A common solvent for reactions and extractions.
- Cosmetics: A key ingredient in nail polish removers.
- Pharmaceuticals: Used in the production of various drugs.
The widespread use of acetone necessitates reliable methods for its identification and quality control, making IR spectroscopy invaluable.
IR Spectroscopy: An Essential Analytical Tool
IR spectroscopy is based on the principle that molecules absorb specific frequencies of infrared radiation.
These frequencies correspond to the vibrational modes of the molecule, such as stretching and bending of bonds.
The resulting absorption spectrum acts as a unique fingerprint, revealing the molecule’s structural features and composition.
Key advantages of IR spectroscopy include:
- Non-destructive Analysis: The sample remains intact after measurement.
- Rapid Identification: Provides quick and reliable identification of compounds.
- Functional Group Information: Reveals the presence of specific chemical groups.
- Versatile Application: Applicable to solids, liquids, and gases.
Objective: A Practical Guide to Acetone IR Spectrum Interpretation
This article aims to provide a comprehensive guide to interpreting the IR spectrum of acetone.
We will explore the characteristic peaks associated with its molecular structure.
By understanding these features, readers will gain the ability to confidently identify acetone and analyze its purity using IR spectroscopy.
This article will serve as a practical resource for students, researchers, and professionals who utilize IR spectroscopy in their work. We will delve into the key vibrational modes and spectral regions that define acetone’s IR signature, offering insights into the underlying molecular behavior.
Acetone, a ubiquitous organic solvent, plays a critical role in various industrial, laboratory, and even household applications. From nail polish remover to chemical synthesis, its versatility is undeniable. Understanding its molecular fingerprint is crucial for quality control, identification, and research purposes.
Infrared (IR) spectroscopy offers a powerful, non-destructive method for probing the vibrational modes of molecules. By analyzing the absorption of infrared radiation, we can gain valuable insights into the functional groups present within a molecule. This information allows us to identify and characterize substances with remarkable accuracy.
Acetone (CH3COCH3), also known as propanone, is the simplest ketone. It is a colorless, volatile, and flammable liquid. Its excellent solvent properties make it ideal for dissolving a wide range of organic materials.
Its applications span across diverse sectors:
- Industrial Solvent: Used in paints, varnishes, resins, and adhesives.
- Laboratory Reagent: A common solvent for reactions and extractions.
- Cosmetics: A key ingredient in nail polish removers.
- Pharmaceuticals: Used in the production of various drugs.
The widespread use of acetone necessitates reliable methods for its identification and quality control, making IR spectroscopy invaluable.
IR spectroscopy is based on the principle that molecules absorb specific frequencies of infrared radiation.
These frequencies correspond to the vibrational modes of the molecule, such as stretching and bending of bonds.
The resulting absorption spectrum acts as a unique fingerprint, revealing the molecule’s structural features and composition.
Key advantages of IR spectroscopy include:
Non-destructive Analysis: The sample remains intact…
The ability of IR spectroscopy to reveal the intricate details of molecular structure makes it an indispensable tool. But to fully appreciate its power, it’s essential to understand the fundamental principles upon which it operates.
The Fundamentals of IR Spectroscopy
At its core, IR spectroscopy is a technique that exploits the interaction between infrared radiation and the vibrational modes of molecules. When a molecule is exposed to IR radiation, it absorbs specific frequencies that correspond to the energies required to excite these vibrations.
This absorption is not random; it’s highly selective, depending on the molecule’s structure and the types of bonds present. The resulting pattern of absorption forms a unique "fingerprint" that can be used to identify and characterize the molecule.
Molecular Vibrations and Wavenumbers
Molecules are not static entities; their atoms are constantly in motion, vibrating around their equilibrium positions. These vibrations can take various forms, including:
- Stretching: Changes in the bond length between two atoms.
- Bending: Changes in the angle between two bonds.
Each vibrational mode has a specific frequency associated with it, determined by the masses of the atoms involved and the strength of the bond.
The frequency of vibration is typically expressed in wavenumbers (cm-1), which is inversely proportional to the wavelength of the absorbed radiation.
Functional Groups: The Key to Interpretation
Specific groups of atoms within a molecule, known as functional groups, exhibit characteristic IR absorption patterns.
For example, a carbonyl group (C=O) typically absorbs strongly in the region of 1650-1800 cm-1, while an alcohol group (O-H) exhibits a broad absorption band around 3200-3600 cm-1.
These characteristic absorptions allow us to identify the presence of specific functional groups within a molecule, providing valuable information about its structure and properties.
Understanding these fundamental principles – the absorption of infrared radiation, the nature of molecular vibrations, and the role of functional groups – is crucial for interpreting IR spectra and extracting meaningful information about molecular structure. By mastering these basics, we can unlock the full potential of IR spectroscopy as a powerful analytical tool.
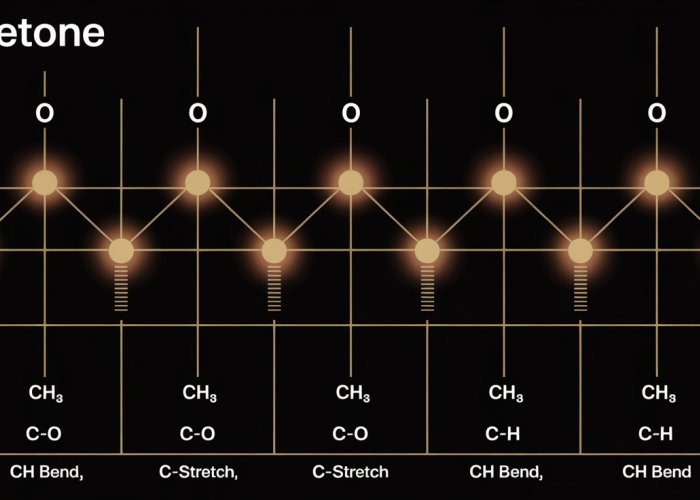
Key Peaks in the Acetone IR Spectrum: A Detailed Analysis
Having established the fundamental principles of IR spectroscopy, we can now turn our attention to the specific features observed in an acetone IR spectrum. A detailed examination of the spectral peaks provides invaluable information about the molecule’s structure and vibrational modes. We will primarily be looking at the C=O stretch and the C-H stretch.
Decoding the Acetone Spectrum: A Focus on Vibrational Modes
The acetone IR spectrum, like a molecular fingerprint, showcases distinct absorption bands. These bands correspond to specific vibrational modes within the acetone molecule. The most prominent peaks arise from the stretching vibrations of the carbonyl group (C=O) and the methyl groups (C-H).
The Carbonyl Stretch (C=O): A Ketone’s Calling Card
The Hallmark Absorption Band
One of the most characteristic features of an acetone IR spectrum is the strong absorption band observed in the region around 1715 cm-1. This band is due to the stretching vibration of the carbonyl group (C=O), a functional group that defines ketones. Its intensity and position make it a key identifier for the presence of a ketone.
Significance in Identifying Ketones
The presence of a strong absorption band in the 1715 cm-1 region is a reliable indicator of a ketone. This distinctive absorption allows for easy identification of acetone and other ketone-containing compounds. However, it’s important to consider other spectral features and chemical knowledge for definitive identification.
Factors Influencing C=O Stretch Position
While the C=O stretch typically appears around 1715 cm-1, its exact position can be influenced by several factors:
-
Inductive Effects: Electron-withdrawing groups near the carbonyl group can increase the wavenumber of the absorption.
-
Resonance Effects: Conjugation of the carbonyl group with a double bond or aromatic ring can decrease the wavenumber.
-
Ring Strain: In cyclic ketones, ring strain can also affect the C=O stretching frequency.
Understanding these effects is critical for accurate interpretation of IR spectra, especially when analyzing more complex molecules.
The Methyl Group’s Contribution: C-H Stretching Vibrations
Absorption Region and Origin
In addition to the prominent C=O stretch, the acetone IR spectrum also exhibits absorptions in the 2900-3000 cm-1 region. These peaks arise from the stretching vibrations of the C-H bonds in the methyl groups (CH3) of acetone. The presence of these peaks confirms the existence of methyl groups within the molecule.
Relation to Methyl Groups
Acetone has two methyl groups, each containing three C-H bonds. The vibrations of these bonds give rise to multiple absorptions in the 2900-3000 cm-1 region. The position and intensity of these peaks depend on the nature of the C-H bonds and their vibrational modes.
Symmetric vs. Asymmetric C-H Stretches
The C-H stretching vibrations can be further classified as symmetric and asymmetric stretches. Symmetric stretches involve the simultaneous stretching of all C-H bonds in a methyl group, while asymmetric stretches involve unsynchronized movements. Asymmetric stretches are typically observed at slightly higher wavenumbers than symmetric stretches. Analysis of these different modes provides more insights into the molecular structure.
Factors Influencing the Acetone IR Spectrum’s Appearance
Having deciphered the fundamental peaks within the acetone IR spectrum, it’s essential to acknowledge that the final spectrum isn’t solely dictated by the molecule itself. Several external factors can subtly or significantly alter the appearance of the spectrum, impacting interpretation and analysis. These factors range from the instrument used to the methodologies employed.
Spectrometer Characteristics and Their Impact
The type of IR spectrometer employed plays a crucial role in shaping the resulting spectrum. Different spectrometers possess varying characteristics that can influence the resolution, sensitivity, and signal-to-noise ratio of the data.
Resolution: Clarity of Spectral Details
Resolution refers to the spectrometer’s ability to distinguish between closely spaced absorption bands. A high-resolution instrument will produce sharper, more well-defined peaks, allowing for the identification of subtle spectral features. Conversely, a low-resolution instrument may broaden peaks, obscuring fine details and potentially leading to misinterpretations.
Sensitivity: Detecting Weak Signals
Sensitivity describes the spectrometer’s capacity to detect weak absorption signals. In situations where the analyte concentration is low or the absorption is inherently weak, a highly sensitive instrument is crucial for obtaining a usable spectrum.
Signal-to-Noise Ratio: Minimizing Interference
The signal-to-noise ratio (SNR) represents the relative strength of the desired signal compared to the background noise. A high SNR indicates a cleaner spectrum with less interference, improving the accuracy and reliability of peak identification. Spectrometers with better noise reduction technologies provide clearer spectra.
The Role of Spectral Databases
Spectral databases are invaluable resources for comparing and identifying unknown compounds based on their IR spectra. These databases contain a vast collection of reference spectra for a wide range of substances.
By comparing an experimental spectrum to those in a database, researchers can quickly narrow down potential matches and confirm the identity of an unknown compound.
However, it’s crucial to remember that database searches should be used as a tool, not a replacement for critical analysis. Factors such as sample purity, instrument variations, and spectral artifacts can influence the accuracy of database matching.
Qualitative Analysis and Data Interpretation
Qualitative analysis of IR spectra involves using the spectral information to identify the presence or absence of specific functional groups and, ultimately, to determine the identity of a compound. This process requires a systematic approach that combines spectral interpretation with chemical knowledge.
Key Steps in Qualitative Analysis
- Baseline Correction: Correct for baseline drift and remove any artifacts.
- Peak Identification: Identify and label all significant peaks in the spectrum.
- Functional Group Assignment: Assign each peak to a specific functional group based on its position and intensity.
- Comparison to Standards: Compare the spectrum to reference spectra or spectral databases.
- Consideration of Chemical Context: Take into account any prior knowledge about the sample, such as its source or method of preparation.
Data Interpretation: A Holistic Approach
Interpreting an IR spectrum is not simply about memorizing peak positions. It requires a holistic understanding of the relationship between molecular structure and vibrational modes.
Factors such as inductive effects, resonance, and hydrogen bonding can all influence the position and intensity of absorption bands. By carefully considering these factors, researchers can gain a deeper understanding of the molecular structure and properties of the analyte.
Acetone IR Spectrum: FAQs
Want to understand your acetone IR spectrum results better? Here are some frequently asked questions to help clarify things.
What does the strong peak around 1715 cm-1 in an acetone IR spectrum indicate?
That strong peak signifies the carbonyl (C=O) stretch. It’s a key indicator of acetone presence. Its location is highly characteristic of the carbonyl group in acetone.
Why are there smaller peaks in an acetone IR spectrum besides the carbonyl peak?
These smaller peaks correspond to other vibrational modes like C-H stretches and bends. While the carbonyl peak is most prominent, these provide additional information about the acetone molecule’s structure. Consider them fingerprints for acetone ir spectrum.
Can I use an acetone IR spectrum to determine the purity of an acetone sample?
Yes, by comparing the relative intensities of the acetone peaks with peaks from potential impurities, you can estimate purity. Absence of expected impurity peaks helps confirm purity.
How does the solvent affect the acetone IR spectrum?
The solvent can influence the peak positions and intensities. Choose a solvent that doesn’t interfere with the acetone peaks. Comparing gas phase acetone ir spectrum with liquid phase ones will show spectral differences.
Hopefully, this helped make sense of that acetone IR spectrum! Let me know if you have any more questions – happy analyzing!